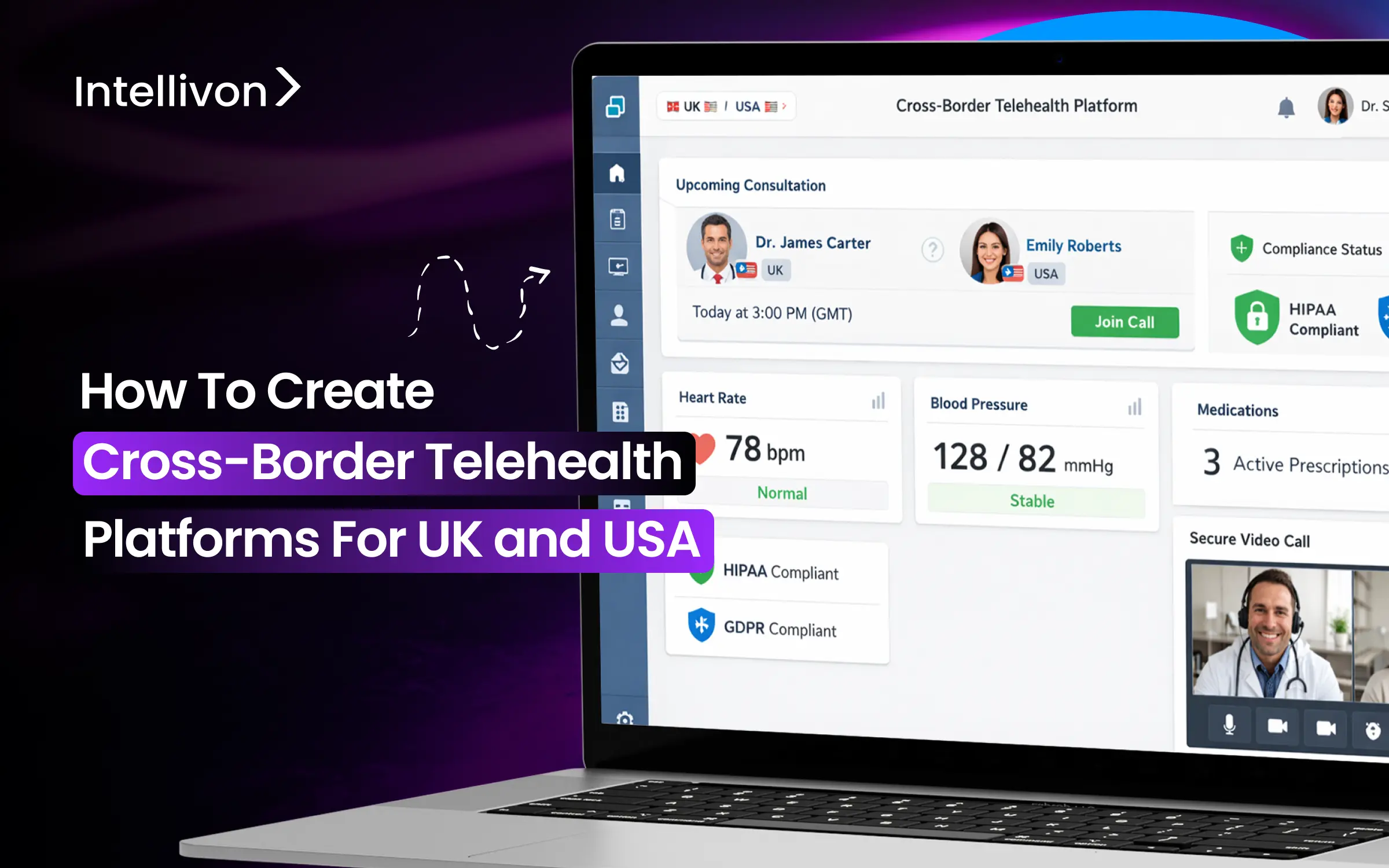
Scaling cross-border telehealth seems simple, but it is more complex than it appears. A platform that works well in one country often shows problems when you expand into another. In these situations, regulatory assumptions do not hold, data residency rules conflict, and responsibility becomes unclear when a UK clinician treats a US patient or the other way around. What seemed like a controlled operation becomes riskier.
This is not just a hypothetical issue. UK providers are entering international markets with private care and direct-to-consumer models. At the same time, US health systems are serving global employees, expats, and patients outside the country. The demand exists, but the systems to provide care across borders without breaking compliance or creating governance gaps often do not.
The solution is to design the platform as a regulatory operating system from the start. This approach ensures accountability regardless of where the clinician or patient is located. We have built enterprise-grade cross-border telehealth platforms that incorporate accountability and AI governance into their core structure. This allows organizations to scale internationally without creating long-term operational or regulatory risks. In this blog, we will share our experience and explain how we built and scaled these platforms from the ground up.
Key Takeaways Of The Cross-Border Telehealth App Market
From an enterprise perspective, cross-border telehealth sits at the intersection of regulated care delivery, international operations, and digital health infrastructure. Unlike domestic telehealth, success depends on jurisdiction-aware platforms that can enforce licensing, prescribing, data protection, and accountability rules automatically as care crosses borders between the UK and the USA.
The global market for cross-border telehealth platforms was valued at approximately USD 4.7 billion in 2024, driven by rising demand for international virtual consultations, specialist second opinions, and remote follow-up care. For healthcare enterprises, this growth reflects a shift from experimental international care programs toward regulated, platform-led delivery models that can scale without increasing compliance risk.
At the same time, the broader telehealth market is projected to reach approximately USD 175.5 billion in 2025 and is expected to grow to over USD 505 billion by 2030, reflecting sustained expansion at an annual growth rate above 23%. As telehealth scales globally, regulatory enforcement and accountability increasingly determine which platforms can operate across borders sustainably.
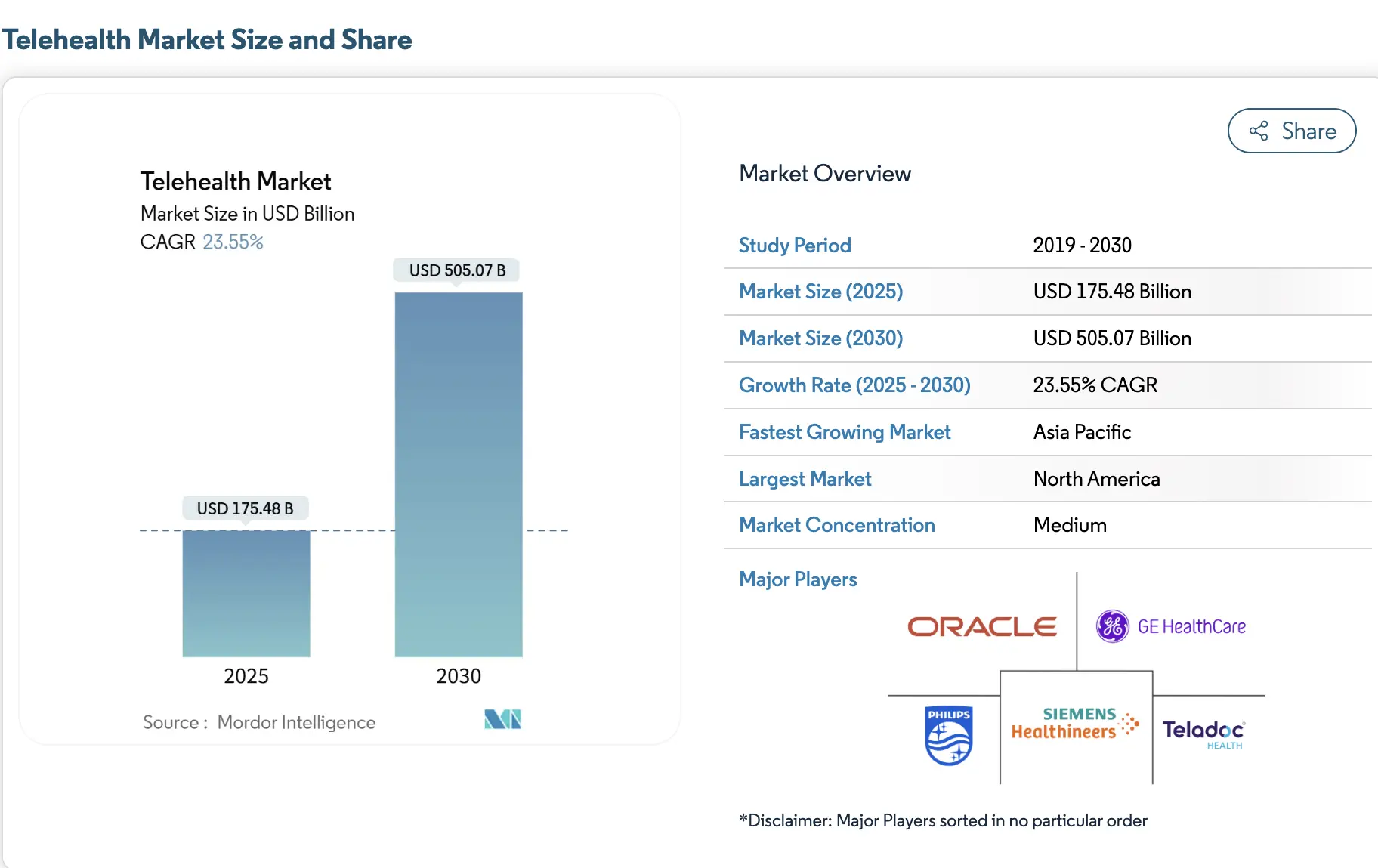
Key Growth Insights:
Several structural forces are accelerating the adoption of cross-border telehealth. Together, they explain why international virtual care is moving from edge use cases to repeatable growth models.
-
Care access gaps: In many regions, specialist availability remains limited. As a result, patients increasingly seek virtual consultations and second opinions from international providers when local options fall short.
-
Economics of medical travel: At the same time, transparent pricing and bundled digital services are making cross-border virtual care more attractive to self-pay patients who want predictable treatment costs without physical travel.
-
Digital readiness: In parallel, broader broadband access, higher smartphone penetration, and AI-driven language support are enabling smoother remote consultations and more secure clinical data exchange across borders.
-
Insurer-led adoption: In addition, global health plans are deploying telehealth networks to support traveler triage and expatriate populations. This approach allows earlier intervention and helps reduce downstream claims.
-
Regulatory evolution: Finally, emerging policy frameworks across Europe, the GCC, and parts of Asia are simplifying cross-border licensing, e-prescribing, and remote care delivery, lowering barriers to scale.
Cross-border telehealth between the UK and the USA often follows a few practical operating models. For example, UK specialist networks provide paid second opinions and structured follow-up care to international patients, generating incremental revenue without physical expansion. Meanwhile, US health systems and insurers use telehealth to support expatriates and travelers, which helps reduce emergency escalations and unnecessary in-person visits.
In both cases, long-term success depends on enforcing licensing, prescribing, and data controls at the platform level. When these rules are embedded into system behavior, organizations can limit compliance risk while enabling scalable, predictable growth.
What are Cross-Border Telehealth Platforms?
Cross-border telehealth platforms enable regulated virtual care delivery across multiple countries, such as the UK and the USA, by enforcing jurisdiction-specific licensing, data protection, prescribing, and accountability rules at the system level. Unlike standard telehealth tools, these platforms separate patient experience from regulatory execution, ensuring care remains compliant regardless of where clinicians or patients are located.
These platforms rely on a cross-border telehealth platform architecture that can detect jurisdiction, enforce regulation, and preserve accountability automatically. In practice, this means regulatory logic is enforced at the system level rather than managed manually by clinicians or administrators.
In a UK–US context, this requires a clear separation between patient experience and regulatory execution. While patients move through a seamless care journey, the platform enforces local clinician licensing, prescribing limitations, consent models, and data residency requirements behind the scenes. As a result, accountability remains clear even as care spans borders.
How Do They Work?
Cross-border telehealth platforms work by separating patient experience from jurisdiction-specific regulatory enforcement. While patients move through a seamless care journey, the platform continuously applies UK or US rules governing licensing, consent, data access, prescribing, and accountability before any clinical action occurs.
Step 1: Patient Intake And Location Detection
First, the platform identifies where the patient is located at the time of care. This step determines which regulatory framework applies and sets the rules for licensing, consent, and data handling before any interaction begins.
Step 2: Identity And Eligibility Verification
Next, patient identity and eligibility are validated using jurisdiction-appropriate checks. This ensures the individual can legally receive care in that location and prevents access from being routed through incorrect regulatory pathways.
Step 3: Clinician matching with licensing controls
Once eligibility is confirmed, clinicians are assigned only if their licensing, scope of practice, and supervisory requirements align with the patient’s location. These constraints are enforced automatically, preventing misalignment before care is delivered.
Step 4: Consent and data access enforcement
At this stage, consent is captured using region-specific requirements. Data access is then restricted to what is permitted under UK or US regulations, ensuring clinicians see only the information they are authorized to view.
Step 5: Clinical workflow and documentation
Care delivery follows jurisdiction-aware workflows, including documentation standards and escalation logic. Importantly, every action is logged to support auditability and clinical accountability across borders.
Step 6: Prescribing and fulfillment control
If prescribing is required, the platform applies country-specific rules. Depending on regulatory limits, actions are permitted, restricted, or routed to local providers or pharmacies.
Step 7: Audit trails and ongoing monitoring
Finally, all decisions, access events, and handoffs are recorded. This creates a continuous audit trail that supports compliance reviews, internal governance, and operational oversight.
Cross-border telehealth platforms succeed by turning regulation into executable logic. By enforcing UK and US rules directly through software, enterprises can scale virtual care safely while maintaining accountability, compliance, and control.
UK vs USA: Regulatory Foundations That Shape Platform Design
Regulation in cross-border telehealth actively shapes how platforms authenticate users, route care, store data, and enforce accountability. Because the UK and the USA apply controls differently, platform architecture must respond accordingly. Otherwise, design decisions introduce risk long before scale becomes visible. In cross-border telehealth, regulatory differences are not edge cases. They are the primary system design constraints.
Is Cross-Border Telehealth Legal Between the UK and the USA?
Yes, cross-border telehealth between the UK and the USA is legal when platforms enforce jurisdiction-specific regulations at the point of care. This includes clinician licensing, prescribing limits, consent requirements, data residency controls, and auditability. Legality depends on how care is governed and executed, not where the platform is hosted.
UK Regulatory Environment
The UK operates under a centrally governed healthcare and data protection model.
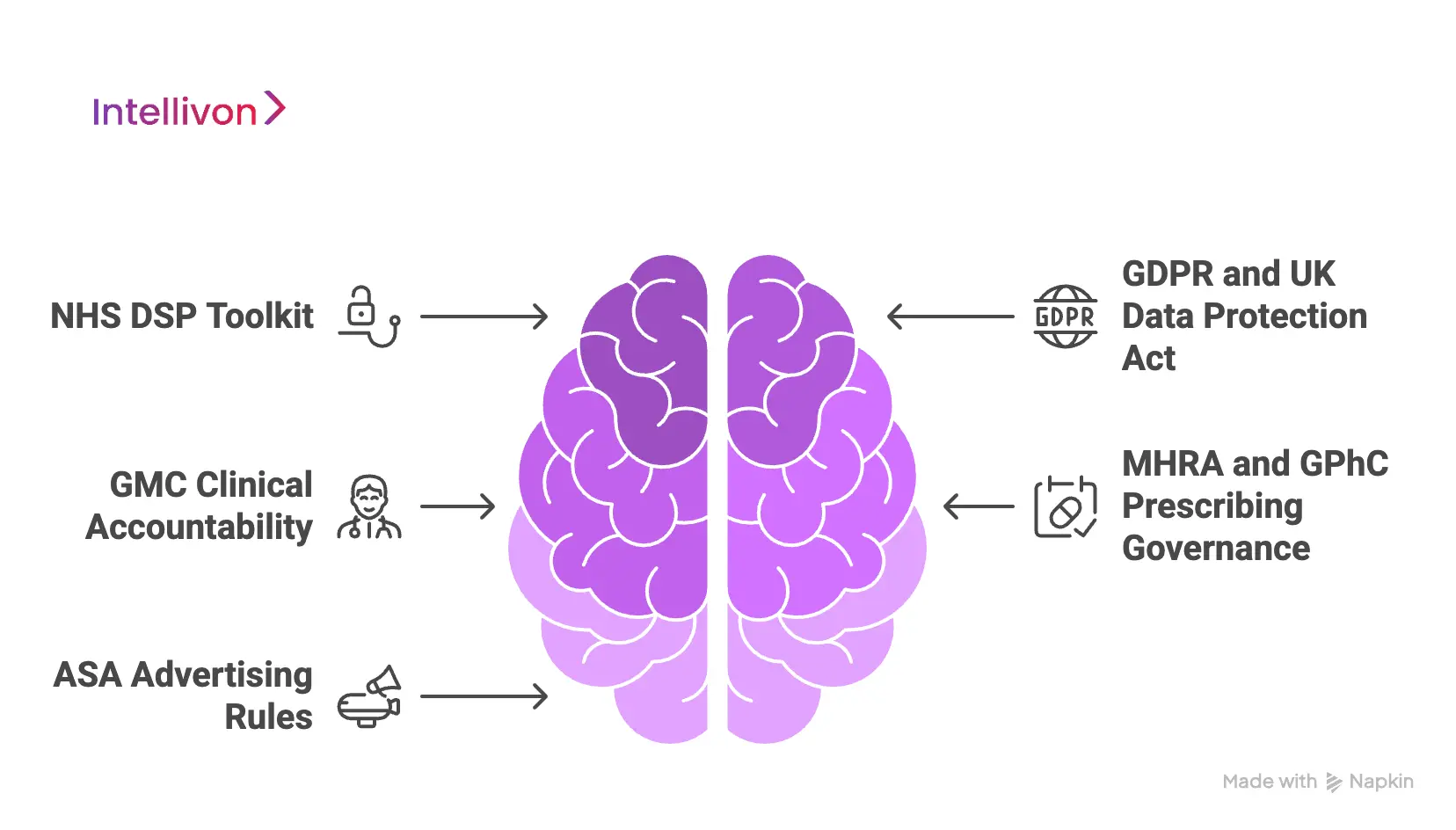
1. NHS DSP Toolkit
The NHS Data Security and Protection Toolkit defines baseline expectations for security posture, supplier governance, and operational controls. Therefore, platforms operating in or alongside UK healthcare must demonstrate structured risk management, controlled access, and defined incident response, even in private or DTC settings. As a result, platforms adopt standardized security architecture rather than ad hoc controls.
2. GDPR and UK Data Protection Act
UK data protection laws tightly regulate consent, purpose limitation, and data minimization. Consequently, platforms must actively enforce who can access patient data, for what reason, and for how long. From a design standpoint, this requires granular permissioning, audit-ready access logs, and consent models that persist across encounters.
3. GMC Clinical Accountability
The General Medical Council assigns clear responsibility to individual clinicians for care decisions. Therefore, platforms must preserve clinician attribution, decision traceability, and escalation visibility. When workflows become shared or opaque, accountability weakens and regulatory exposure increases.
4. MHRA and GPhC Prescribing Governance
Medication prescribing and fulfillment operate under strict controls. As a result, platforms must enforce prescribing boundaries, supervision rules, and pharmacy integration logic at the system level. These requirements limit unchecked automation and demand explicit workflow enforcement.
5. ASA Advertising Rules
UK advertising standards require digital health claims to remain accurate and defensible. Consequently, platforms must govern not only marketing content but also in-app messaging, patient education, and automated communications. This ensures representations remain compliant across channels
US Regulatory Environment
The US regulatory landscape follows a more distributed and enforcement-driven model. Therefore, platforms must adapt dynamically based on state, service type, and risk profile.

1. HIPAA and HITECH
US privacy regulations focus heavily on protecting health information and enforcing breach accountability. As a result, platforms must implement strong access controls, encryption, and continuous monitoring. Enforcement typically occurs retrospectively, which makes evidence generation essential.
2. State-Based Licensure
Licensure in the US varies by state rather than nationally. Therefore, platforms must dynamically match patients with clinicians licensed in the correct jurisdiction at the time of care. This introduces routing complexity that systems must enforce automatically to avoid violations.
3. DEA and Ryan Haight Act
Remote prescribing, especially for controlled substances, faces strict limitations. Consequently, platforms must validate eligibility, enforce encounter requirements, and restrict prescribing actions when conditions are unmet. Manual review does not scale in this environment.
4. FDA SaMD Guidance
Depending on functionality, clinical software may fall under Software as a Medical Device oversight. As a result, platforms must design, validate, and update decision support and AI-assisted workflows carefully to remain compliant.
5. FTC Health Data Enforcement
The FTC increasingly regulates how organizations collect, share, and monetize health data. Therefore, platforms must treat secondary data use, analytics, and third-party integrations as regulated activities rather than optional considerations.
These differences are why successful cross-border telehealth platforms are designed as regulatory operating systems rather than extensions of domestic telehealth tools.
62% Out-Of-State Telemedicine Users Had Prior In-Person Visits
Cross-border telehealth is often misunderstood as a first-touch care channel. In practice, the opposite is true. Primary data shows that 62.6% of out-of-state telemedicine visits involved patients who had already seen the same clinician in person.
This pattern matters because regulators evaluate remote care differently when it extends an existing clinical relationship rather than initiating treatment without prior context. That prior relationship carries real weight in both clinical decision-making and regulatory defensibility. This insight changes how cross-border platforms should be designed.
1. Continuity Reduces Clinical and Legal Risk
When a clinician already knows the patient, remote care becomes an extension of care and not a replacement. Clinical context exists, medical history is trusted, and escalation decisions are clearer.
From a regulatory standpoint, this continuity strengthens accountability and reduces exposure to duty-of-care and prescribing decisions. For enterprises, this lowers risk without slowing access.
2. Cross-Border Care Often Starts After the First Visit
Many successful cross-border models do not begin with virtual-first encounters. They begin after diagnosis, treatment planning, or an initial consultation has already occurred locally.
Telehealth then supports follow-ups, second opinions, monitoring, and continuity across geography. This pattern aligns with how regulators evaluate responsibility.
3. Platform Design Must Preserve Relationship Context
To support this reality, platforms must retain and surface prior encounter history across borders. Identity resolution, clinician attribution, and longitudinal records cannot break when geography changes.
Without this, continuity exists in theory but disappears in execution. This is where many platforms fail quietly.
Cross-border telehealth scales safely when platforms preserve continuity and attribution, not when they maximize virtual visit volume.
Core Platform Architecture for UK–US Cross-Border Telehealth
Cross-border telehealth architecture exists to enforce rules at scale. In the UK–US context, platforms must deliver a unified care experience while applying different regulatory, clinical, and data controls depending on where care occurs.
Platforms that rely on policy, training, or post-hoc review to manage cross-border compliance accumulate risk as scale increases. This requires a layered system where governance is embedded into core workflows rather than managed through policy or manual oversight.
1. Experience and Access Layer
This layer handles patient-facing interactions such as onboarding, scheduling, and communication. It remains consistent across regions to avoid fragmented user journeys.
However, it does not make regulatory decisions. By keeping experience separate from enforcement, platforms can evolve user interfaces without reworking compliance logic.
2. Jurisdiction Detection and Policy Layer
The platform continuously determines where care is delivered and which regulatory framework applies. Patient location, clinician location, and service type are evaluated in real time.
This layer activates UK or US rules before any clinical action occurs, preventing misrouted care and downstream violations.
3. Identity and Access Governance
Identity verification and role-based access control ensure that only authorized users can act within the system. Clinicians are matched based on licensing and scope of practice, while patients are authenticated using region-appropriate checks.
This layer protects against unauthorized access and supports audit readiness.
4. Clinical Workflow Orchestration
Care delivery follows structured workflows that adapt to jurisdictional rules. Documentation standards, escalation paths, and supervision requirements are enforced automatically. This ensures accountability remains clear even as care spans borders.
5. Prescribing and Fulfillment Controls
Prescribing logic is tightly governed. The platform enforces country-specific rules, blocking or routing actions that fall outside permitted boundaries. Pharmacy integrations operate within local regulations, reducing legal exposure while maintaining continuity of care.
6. Data Residency and Sovereignty Enforcement
Patient data is stored, accessed, and processed according to regional requirements. The platform restricts cross-border data movement while allowing permitted insights to flow through governed channels. This protects privacy without limiting operational visibility.
7. AI Governance and Decision Support
AI assists intake, documentation, and risk flagging, but never replaces clinical accountability. Decision support is explainable, jurisdiction-aware, and subject to human oversight.
This prevents uncontrolled automation in regulated environments.
8. Audit, Logging, and Compliance Evidence
Every action, access event, and decision is logged with traceability. This creates a continuous audit trail that supports regulatory reviews, internal governance, and incident response across both markets.
UK–US cross-border telehealth platforms succeed when architecture enforces regulation by design. By separating experience from governance and encoding rules into system behavior, enterprises can scale international care without sacrificing control, compliance, or long-term flexibility. Enterprises that adopt this architecture avoid rework, regulatory remediation, and stalled expansion as cross-border programs grow.
Key Features Required in a Cross-Border Telehealth Platform
Cross-border telehealth platforms succeed when features are designed to control risk while enabling growth. In a UK–US context, functionality must adapt to where care is delivered, who delivers it, and what actions are permitted.
The following features are foundational for enterprises building regulated, scalable international care systems.
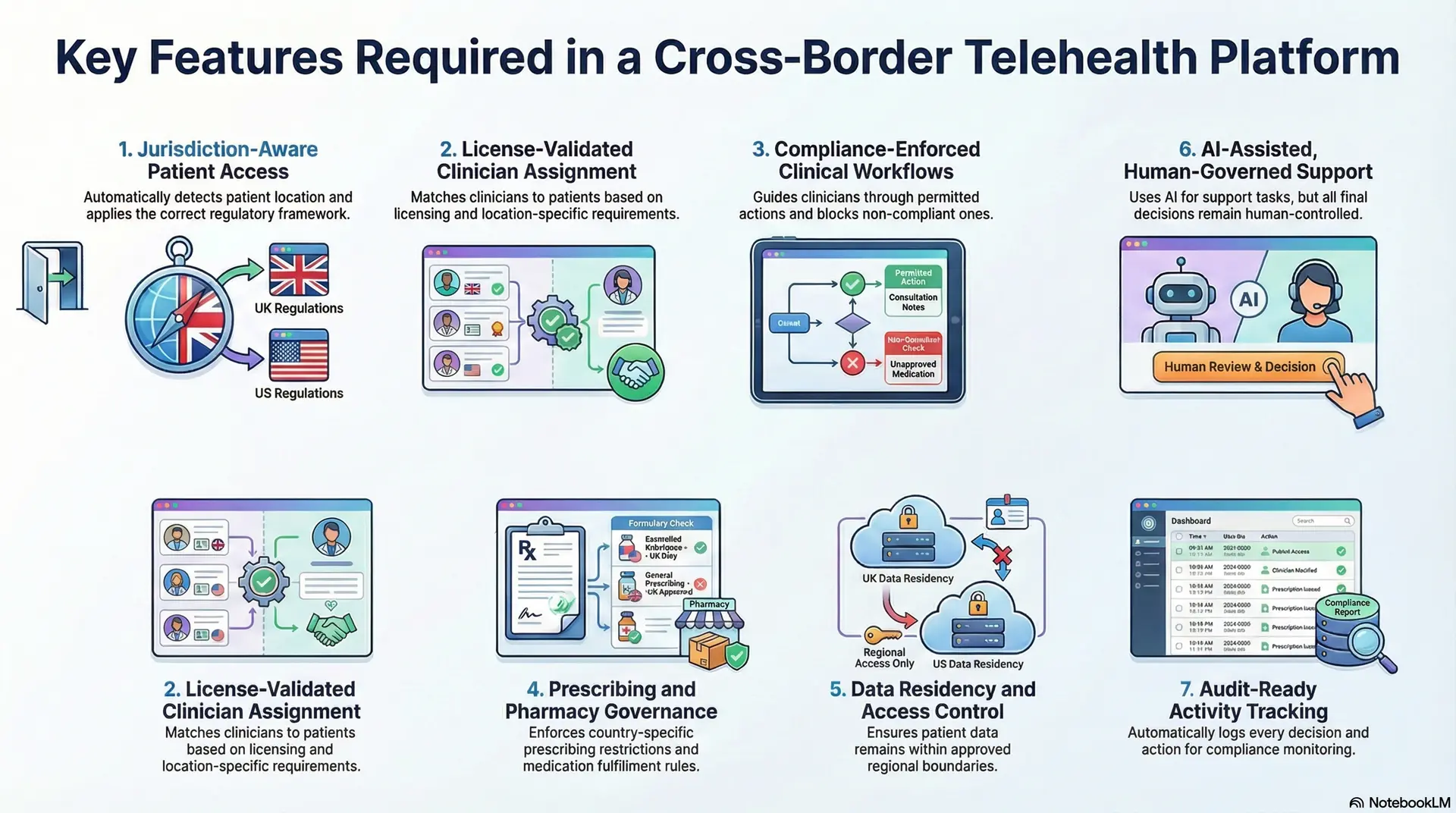
1. Jurisdiction-Aware Patient Access
The platform must detect patient location at the time of care and apply the correct regulatory framework automatically.
Intake, consent, and service eligibility adjust based on whether care occurs in the UK or the US. Without this, access becomes inconsistent, and compliance exposure increases.
2. License-Validated Clinician Assignment
Clinicians should only be matched to patients when their licensing, scope of practice, and supervision requirements align with the patient’s location.
This feature prevents cross-border misrouting and protects organizations from unauthorized care delivery.
3. Compliance-Enforced Clinical Workflows
Clinical workflows must encode local documentation standards, escalation paths, and accountability requirements.
The platform should guide clinicians through permitted actions while blocking or rerouting those that violate jurisdiction-specific rules.
4. Prescribing and Pharmacy Governance
Prescribing logic must respect country-specific restrictions and fulfillment pathways. The platform enforces what can be prescribed, when in-person follow-up is required, and how medications are dispensed, reducing legal and clinical risk.
5. Data Residency and Access Control
Patient data must remain within approved regional boundaries, with access restricted by role and purpose. This ensures privacy obligations are met without limiting necessary clinical visibility.
6. AI-Assisted, Human-Governed Support
AI can support intake, documentation, and risk identification, but decisions must remain traceable and human-controlled. Clear boundaries prevent automation from creating regulatory ambiguity.
7. Audit-Ready Activity Tracking
Every access, decision, and handoff should be logged automatically. This enables continuous compliance monitoring and simplifies regulatory review across both markets.
In cross-border telehealth, features are not conveniences. They are enforcement mechanisms. Platforms that embed these capabilities at the core are easier to govern, safer to scale, and better positioned for long-term growth across the UK and the USA.
AI in Cross-Border Telehealth Platforms: Practical, Governed Use
In cross-border telehealth, AI supports coordination and compliance, not autonomous clinical decision-making, with human accountability enforced across UK and US regulations. In regulated healthcare, AI maturity is measured by control and traceability, not autonomy.
It must function as a controlled support layer that improves consistency, reduces manual burden, and strengthens oversight without weakening accountability.
1. Where AI Delivers Proven Value
AI is most effective when applied to operational and preparatory tasks. It can structure patient intake, summarize clinical histories, flag risk indicators, and support documentation quality.
These uses reduce clinician workload and improve accuracy without altering who is responsible for care decisions. Because outputs are reviewable and traceable, they align well with regulatory expectations in both markets.
2. Using AI to Enforce Consistency at Scale
As cross-border programs grow, variation becomes a risk. AI helps standardize how information is captured, how cases are triaged, and how workflows progress across regions. This consistency is especially important when UK and US rules differ, as it prevents informal workarounds and keeps execution aligned with policy.
3. Where AI Introduces Risk
AI becomes problematic when it moves into diagnosis generation, autonomous triage, or prescribing recommendations. These areas raise questions around liability, explainability, and regulatory approval.
In cross-border contexts, the risk compounds because rules differ by jurisdiction, and accountability must remain local.
4. Human Oversight as a System Requirement
Successful platforms design human-in-the-loop oversight into architecture, not policy. AI outputs are clearly labeled, reviewable, and interruptible.
Final decisions remain attributable to licensed clinicians, preserving trust with regulators and care teams.
In cross-border telehealth, AI maturity is measured by control and clarity, not autonomy. Platforms that use AI to strengthen governance and execution can scale safely across the UK and the USA.
How We Build Cross-Border Telehealth Platforms For UK and USA
At Intellivon, we do not treat cross-border telehealth as a feature rollout. We treat it as enterprise platform engineering. Our build approach is designed to absorb regulatory complexity early, so scale does not introduce instability later.
Each step is deliberately sequenced to reduce risk, control cost, and support long-term growth across the UK and the USA.
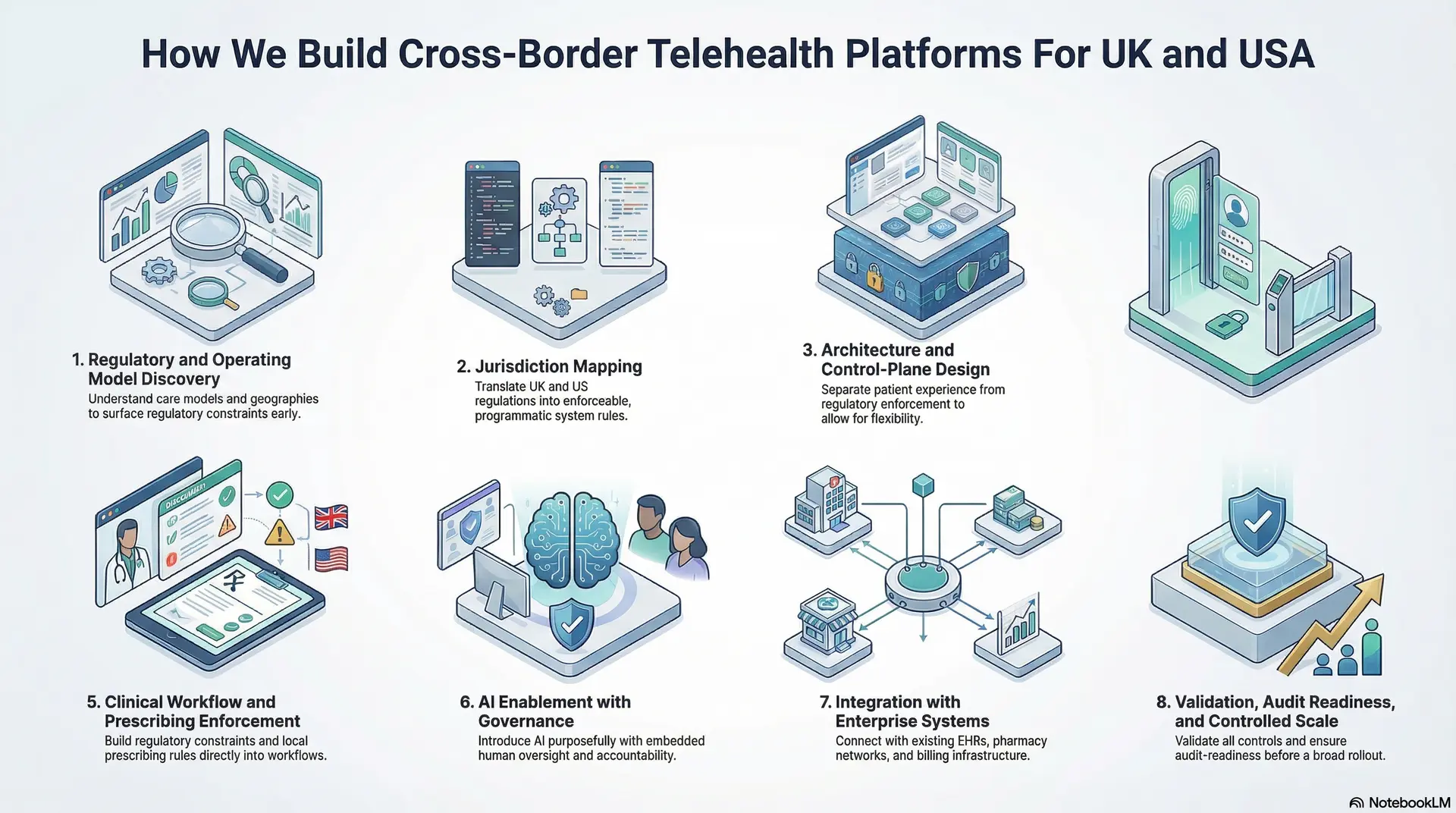
Step 1: Regulatory and Operating Model Discovery
We start by understanding how care is intended to operate, not just where it will launch. This includes care models, target geographies, clinician structures, prescribing needs, and patient journeys.
We assess whether care is episodic, longitudinal, or hybrid, and how accountability is expected to flow across borders. This step surfaces regulatory constraints early, before architectural decisions lock the platform into risky assumptions.
Step 2: Jurisdiction Mapping
Next, we translate UK and US regulatory requirements into explicit system rules. Licensing boundaries, consent requirements, prescribing limits, supervision needs, and data access policies are mapped by jurisdiction.
These are defined so they can be enforced programmatically, ensuring consistency as the platform scales.
Step 3: Architecture and Control-Plane Design
We design a layered architecture that separates patient experience from regulatory enforcement. The control plane governs identity, jurisdiction logic, workflows, and permissions, while the experience layer remains flexible.
This separation allows the platform to evolve without breaking compliance when regulations change or new regions are added.
Step 4: Identity, Access, and Accountability Setup
Identity is treated as foundational infrastructure. Clinicians, patients, and administrators are verified using jurisdiction-appropriate methods. Roles and permissions are tightly scoped, ensuring users can only perform actions they are legally authorized to take.
Clinician attribution and decision traceability are preserved across all workflows to support accountability.
Step 5: Clinical Workflow and Prescribing Enforcement
Clinical workflows are implemented with built-in regulatory constraints. Documentation standards, escalation logic, and supervision requirements vary by jurisdiction and are enforced automatically.
Prescribing actions are permitted, restricted, or rerouted based on local rules, reducing reliance on manual checks and minimizing exposure.
Step 6: AI Enablement with Governance
AI is introduced carefully and purposefully. It supports intake structuring, documentation quality, and risk flagging, while remaining fully explainable and interruptible.
Human oversight is embedded into workflows, so accountability never shifts away from licensed clinicians. This approach allows AI to scale operations without introducing regulatory ambiguity.
Step 7: Integration with Enterprise Systems
The platform is integrated with existing EHRs, pharmacy networks, identity providers, analytics systems, and billing infrastructure where required.
These integrations preserve continuity across care delivery, operations, and reporting, ensuring the platform fits into the broader enterprise ecosystem rather than operating in isolation.
Step 8: Validation, Audit Readiness, and Controlled Scale
Before scaling, we validate workflows against real regulatory scenarios in both the UK and the USA. Audit trails, access logs, and reporting mechanisms are reviewed to ensure evidence is regulator-ready.
Only once controls are proven do we support broader rollout, reducing the likelihood of costly remediation later.
By embedding compliance, governance, and accountability into every stage of delivery, we help enterprises expand across the UK and the USA without sacrificing control, trust, or long-term flexibility.
Cost to Build a Cross-Border Telehealth Platform (UK + USA)
Cost in cross-border telehealth platforms is driven by governance complexity and jurisdiction scope, not interface design alone. Most enterprises begin with a focused platform that supports jurisdiction-aware consultations, clinician licensing controls, secure data handling, and limited prescribing or follow-up care. The platform then expands as demand, regulatory confidence, and operational maturity grow.
At Intellivon, we structure cost around what must be governed on day one versus what can safely evolve later. This approach helps organizations enter cross-border care quickly, validate real usage, and avoid overbuilding features before they deliver measurable value.
Estimated Cost Breakdown (USD 50,000–150,000)
| Cost Component | What It Covers | Estimated Range |
| Cross-Border Discovery & Care Model Design | UK–US use cases, service scope, regulatory mapping, and platform blueprint | $5,000 – $12,000 |
| Jurisdiction-Aware UX & Intake Flows | Patient onboarding, location detection, consent logic, and eligibility checks | $6,000 – $14,000 |
| Identity, Licensing & Access Controls | Clinician licensing validation, role-based access, patient verification, and audit logs | $6,000 – $15,000 |
| Core Clinical Workflow Engine | Consultation flows, documentation standards, escalation paths, and accountability | $8,000 – $20,000 |
| Prescribing & Fulfillment Logic (Limited Scope) | Jurisdiction checks, prescribing constraints, and routing to local providers or pharmacies | $6,000 – $18,000 |
| Data Handling & Residency Safeguards | UK–US data separation, access enforcement, consent-bound visibility | $5,000 – $12,000 |
| AI-Assisted Intake & Documentation | Structured intake, clinical summaries, risk flags with human oversight | $5,000 – $12,000 |
| Basic Enterprise Integrations | Calendar, identity providers, secure messaging, lightweight EHR connectivity | $5,000 – $15,000 |
| Security, Compliance & Monitoring | GDPR and HIPAA safeguards, logging, monitoring, and incident readiness | $4,000 – $10,000 |
| Testing, Pilot & Stabilization | Regulatory scenario testing, pilot rollout, workflow validation | $3,000 – $8,000 |
Typical Investment Ranges
Lean Cross-Border MVP: $50,000 – $90,000
Best suited for consultative care, second opinions, or follow-up models with limited prescribing and a small clinician cohort. Ideal for early validation across the UK and selected US states.
Enterprise-Ready Phase 1 Platform: $100,000 – $150,000
Supports broader clinician participation, stronger audit readiness, limited prescribing workflows, AI-assisted operations, and deeper operational control across both markets.
Actual cost depends on how much automation, prescribing, and integration are required at launch.
Factors That Influence the Cost of Cross-Border Telehealth Platforms
Cost is driven by governance complexity and jurisdiction scope, not interface design alone.
- Jurisdiction Coverage: Supporting a limited number of US states costs significantly less than nationwide licensure coverage.
- Care Model Scope: Consult-only or follow-up care platforms are leaner. Medication management and controlled substances add complexity.
- Compliance Depth: Basic safeguards are affordable. Fine-grained audit controls and evidence reporting increase effort but protect scale.
- AI Usage” Assistive AI keeps costs low. Advanced orchestration and automation raise investment but improve operational efficiency later.
- Integration Requirements: Standalone platforms launch faster. Deep EHR or payer integrations increase cost but enable enterprise rollout.
When scoped correctly, a cross-border telehealth platform becomes a controlled growth asset, not a financial risk. The key is investing early in the layers that enforce trust, compliance, and accountability, rather than paying for remediation after scale exposes gaps.
Common Mistakes Enterprises Make When Scaling Cross-Border Telehealth
Scaling cross-border telehealth exposes structural weaknesses that are easy to miss during early pilots. Platforms that appear stable in a single market often struggle once extended across the UK and the USA. At first, these issues remain subtle. Over time, however, they surface as audit friction, clinician hesitation, stalled expansion, or rising regulatory exposure. Most cross-border telehealth failures are architectural, not operational. They emerge only after scale exposes governance gaps.
Understanding where platforms break down helps enterprises avoid repeating the same patterns.
1. Treating Compliance as Documentation
Many organizations rely on policies, training manuals, and internal checklists to manage cross-border compliance. Initially, this feels sufficient. However, as patient volumes grow and workflows multiply, manual enforcement becomes inconsistent. Gaps begin to form between how care is designed to operate and how it actually functions day to day.
To address this, regulatory requirements must move beyond documentation. Our teams translate licensing rules, consent logic, prescribing boundaries, and data access policies into enforceable system behavior. As a result, compliance no longer depends on memory or interpretation.
2. Reusing Single-Country Workflows
Another common shortcut is extending existing telehealth workflows into new regions with minimal change. While this speeds early expansion, it introduces hidden risk. UK and US regulations differ materially in clinician accountability, prescribing authority, and data governance. When workflows are reused, responsibility blurs and exposure increases.
In contrast, jurisdiction-aware systems adapt automatically based on where care is delivered. This approach preserves local compliance while allowing platforms to scale without fragmentation or duplication.
3. Losing Clinician Accountability at Scale
As platforms grow, accountability can erode quietly. Shared queues, pooled inboxes, and generic documentation make it harder to trace who made which decision and why. Over time, this weakens both clinical confidence and audit defensibility.
For this reason, accountability must be treated as infrastructure. Identity, access, and documentation are tightly linked so that every clinical action remains attributable, traceable, and defensible across borders.
4. Allowing AI to Drift Beyond Governance
AI is often introduced with a narrow intent, such as improving intake or documentation. Over time, however, it may expand into higher-risk areas without sufficient oversight. In cross-border care, this creates regulatory ambiguity and raises questions around liability and explainability.
Based on experience in regulated environments, AI must be constrained by design. Clear boundaries ensure AI assists with intake, documentation, and risk signals, while final decisions remain human-led, reviewable, and jurisdiction-aware.
5. Not Respecting Data Residency Rules
To simplify reporting and analytics, some platforms centralize patient data across regions. Although convenient, this approach conflicts with GDPR, HIPAA, and emerging enforcement trends. As a result, long-term compliance risk increases.
A more durable approach enforces regional data residency while still enabling governed, policy-aware insights. This allows leadership to maintain visibility without violating local requirements.
6. Scaling Before Audit Readiness Is Proven
Finally, enterprises sometimes expand into new markets before validating audit trails and evidence generation. In these cases, issues only surface when regulators, partners, or insurers request proof of compliance. By then, remediation is costly and disruptive.
Validating audit readiness early changes this dynamic. Before scale, workflows are tested against real regulatory scenarios to ensure logs, controls, and reporting hold up under scrutiny.
Enterprises that address these issues early build cross-border telehealth platforms that scale with confidence. Those who delay enforcement often pay for it later through lost momentum, higher remediation costs, and increased regulatory risk. Most of these failures only become visible after scale, when reversing architectural decisions becomes expensive and disruptive.
Conclusion
Cross-border telehealth is no longer a peripheral capability. Instead, it is becoming a core part of how healthcare organizations extend care, protect access, and grow responsibly across the UK and the USA. As digital care models mature, international delivery is moving from exception to expectation.
However, platforms that succeed are not defined by speed alone. They are shaped by how effectively accountability, compliance, and operational control are enforced as services scale across jurisdictions. Without these foundations, growth introduces risk rather than resilience.
When built correctly, cross-border telehealth becomes a growth enabler rather than a risk surface. For this reason, Intellivon helps enterprises design and build compliance-first telehealth platforms that scale with confidence, preserve regulatory trust, and support long-term expansion across borders. For healthcare enterprises, cross-border telehealth is no longer a technology decision. It is a governance and operating model decision that directly affects risk, trust, and scalability.
For enterprises already operating across multiple regions, the challenge is no longer whether cross-border telehealth is viable, but whether the platform can enforce compliance, accountability, and control as complexity increases.
Build A Compliance-First Cross-Border Telehealth Platform With Intellivon
At Intellivon, we build cross-border telehealth platforms as enterprise operating systems, not virtual care tools stretched across incompatible regulatory environments. From the outset, our platforms are designed to govern how care is delivered, how accountability is enforced, and how compliance is maintained across the UK and the USA. As programs expand across regions and service lines, this governance remains consistent rather than weakening over time.
To support organizations operating at scale, each solution is engineered with architecture as the foundation. Platforms are compliance-led and jurisdiction-aware, with regulatory logic embedded across identity, workflows, data handling, and AI governance. As a result, when cross-border programs grow across patient populations, clinician networks, and regulatory boundaries, control, auditability, and operational predictability remain intact.
Why Partner With Intellivon?
-
Enterprise-grade platform architecture aligned with UK–US regulatory enforcement and scalable cross-border care delivery
-
Deep interoperability expertise across EHRs, pharmacy networks, identity systems, analytics platforms, and enterprise infrastructure
-
Compliance-by-design delivery supporting audit readiness, clinician accountability, data residency, and jurisdiction-specific controls
-
AI-assisted orchestration that improves intake, documentation, and operational efficiency without removing clinical responsibility
-
Proven enterprise delivery model with phased rollout, regulatory validation, and controlled cross-border expansion
Talk to Intellivon’s healthcare platform architects to explore how a compliance-first cross-border telehealth platform can integrate into your existing ecosystem, reduce regulatory risk, and enable sustainable international care delivery with confidence.
FAQs
Q1. What is a cross-border telehealth platform?
A1. A cross-border telehealth platform enables regulated virtual care delivery across multiple countries, such as the UK and the USA. In practice, these platforms enforce local licensing, data protection, prescribing, and clinical accountability rules. As a result, unlike standard telehealth apps, jurisdiction-specific controls are applied automatically at the system level rather than managed manually.
Q2. Is cross-border telehealth legal between the UK and the USA?
A2. Yes, cross-border telehealth is legal between the UK and the USA when platforms enforce jurisdiction-specific regulations. This includes clinician licensing, prescribing limits, data residency, consent requirements, and auditability. Importantly, legality depends on how care is delivered and governed, not simply where the platform is hosted.
Q3. What are the biggest challenges in building cross-border telehealth platforms?
A3. The biggest challenges include enforcing clinician licensing across jurisdictions, managing different prescribing rules, maintaining data residency compliance, preserving clinical accountability, and governing AI usage. Over time, these challenges become more complex as platforms scale beyond pilot programs and operate across multiple regions.
Q4. How much does it cost to build a cross-border telehealth platform?
A4. A focused cross-border telehealth platform for the UK and the USA typically costs between $50,000 and $150,000. However, the final cost depends on jurisdiction coverage, prescribing scope, integration depth, and compliance requirements. As automation, analytics, and regulatory complexity increase, investment requirements also rise.
Q5. How do enterprises ensure compliance in cross-border telehealth?
A5. Enterprises ensure compliance by embedding regulatory enforcement directly into platform architecture. This includes jurisdiction detection, license-validated clinician routing, consent-bound data access, prescribing controls, audit trails, and human-governed AI workflows. Ultimately, compliance must be enforced by software behavior rather than policy documents alone. Enterprises that rely on policy or training alone often encounter compliance gaps once patient volume and clinician participation increase.