Telehealth platforms have largely nailed remote access. However, they still struggle with closure. As a result, care journeys often break at the exact point where evidence should guide the next clinical decision. Telehealth systems that operate without integrated diagnostics can initiate care, but they cannot reliably complete it at scale.
This is where virtual lab integration becomes essential. Rather than acting as a supporting feature, it functions as an infrastructure that governs how diagnostics are ordered, collected, interpreted, and acted upon within the telehealth system itself. In practice, effective integration requires more than APIs. Instead, it demands orchestration across clinicians, labs, logistics partners, data pipelines, and compliance controls, all operating inside a single, accountable workflow.
At this point, platform design becomes the differentiator. Intellivon architects virtual lab integration as core telehealth infrastructure, building closed-loop diagnostic workflows that govern ordering, collection, results delivery, and clinical follow-up within a single platform. We handle the full technical stack, from HL7 and FHIR interoperability to compliance pipelines and orchestration layers that connect clinicians, labs, and logistics into unified, accountable care workflows. This blog draws from that experience and explains how we integrate virtual labs into telehealth systems built to scale safely and predictably.
Why Enterprises Are Investing In Virtual Lab-Enabled Telehealth Now
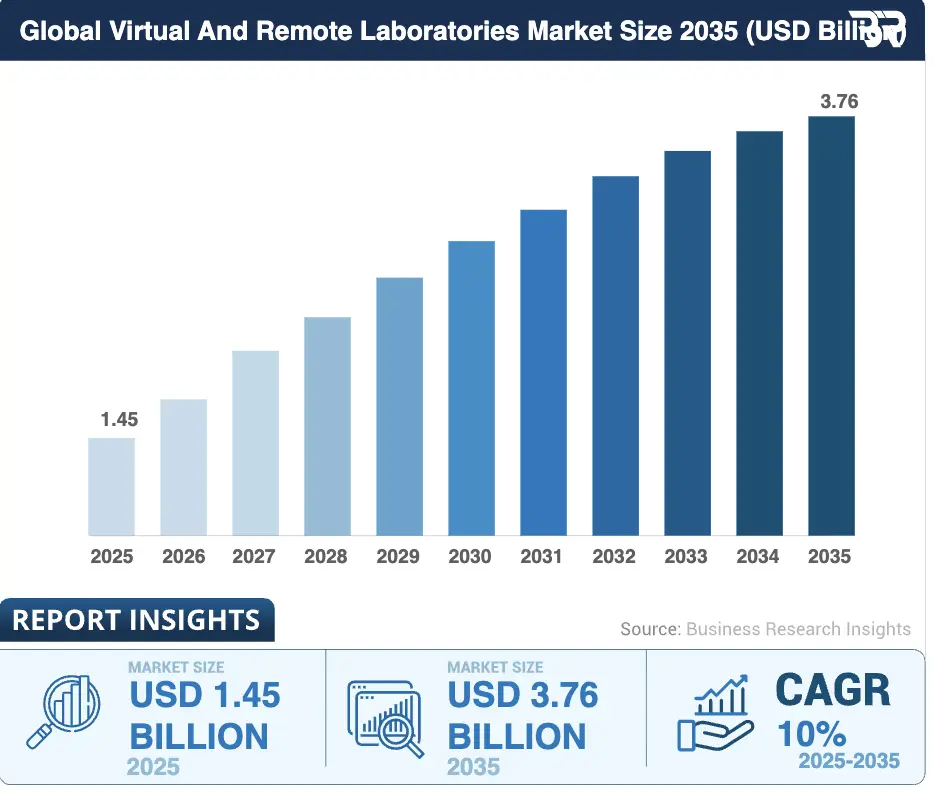
The global market for virtual and remote laboratories was valued at approximately USD 1.45 billion in 2025 and is projected to reach USD 3.76 billion by 2035, reflecting a compound annual growth rate of about 10% over the period.
Market Insights:
Virtual Labs Drivers
- Rising demand for remote collaboration and training simulations is growing the market from USD 1.08B in 2025.
- Lab automation, AI analytics, and cybersecurity enhancements for efficient diagnostics.
- Expansion in telemedicine integration, surgical training, pain management, and cost-reduced hardware.
- Adoption in academic centers for credentialing and pharma-custom modules, cutting onboarding costs.
Telehealth Apps Drivers
- Chronic disorders prevalence and remote patient monitoring needs via AI, IoT, and 5G.
- Smartphone proliferation, high-speed internet, wearables for real-time vitals, and relaxed regulations.
- Favorable reimbursements, government digitization initiatives, and post-COVID hybrid care acceptance.
As these forces converge, enterprises are moving beyond experimentation and investing in virtual lab-enabled telehealth as a scalable, accountable care delivery model. Here is why enterprises are investing in virtual lab-enabled telehealth now:
1. Diagnostic Gaps Limit Telehealth ROI
Telehealth platforms perform well during the consultation. However, value often leaks once diagnostics move outside the system. When lab follow-ups rely on manual tracking or external portals, care journeys slow down and patient engagement drops.
As a result, enterprises see repeat consultations and avoidable in-person escalation. What should resolve in a single episode stretches across multiple touchpoints, increasing cost while reducing operational efficiency and measurable ROI.
2. Virtual Labs Unlock New Care Models
Once diagnostics are embedded into the platform, telehealth can support more than one-time visits. Chronic condition management becomes structured, with recurring labs enabling timely adjustments and continuous oversight rather than reactive intervention.
In addition, preventive screening and employer-led health programs become operationally viable. Virtual labs provide consistent diagnostic data, allowing enterprises to run outcome-driven population health initiatives without adding workflow complexity.
3. Revenue, Retention, And Clinical Completion
Integrated lab workflows improve care completion by connecting consultation directly to evidence-based action. When diagnostics flow inside the platform, fewer journeys stall, and decisions are guided by timely results rather than assumptions.
This continuity strengthens both retention and reimbursement alignment. Completed care episodes are easier to document, audit, and bill, giving enterprises greater financial predictability while preserving clinical accountability.
Taken together, these factors explain why diagnostics now sit at the center of telehealth strategy. Without integrated diagnostics, telehealth struggles to deliver consistent outcomes or predictable returns. Virtual labs restore continuity, accountability, and scalability across the care journey.
What “Virtual Lab Integration” Actually Means In Telehealth
Virtual lab integration refers to the seamless embedding of diagnostic workflows into a telehealth platform so that testing, results, and follow-up operate as part of a single care system. Diagnostics are treated as a core service layer, and not a separate downstream activity.
In a telehealth context, this means lab ordering is initiated during the clinical encounter and automatically routed through approved partners. Sample collection, logistics, and result delivery are coordinated within the platform, allowing clinicians to review findings without leaving their workflows.
Results return as structured data that trigger next steps, including follow-ups, treatment adjustments, or escalation when required. At the same time, consent, access controls, and audit trails are enforced continuously, preserving clinical accountability.
When implemented correctly, virtual lab integration connects evidence to action. It ensures care progresses reliably from consultation to outcome, even as telehealth platforms scale across users, services, and regions.
Core Components Of A Virtual Lab Layer
A virtual lab layer functions as the connective tissue between clinical intent and diagnostic execution. To support telehealth at scale, this layer must coordinate multiple systems while preserving accountability, data integrity, and predictable workflows.

1. Lab Ordering & Routing Engine
The diagnostic journey begins at the point of clinical intent. This component enables clinicians to order diagnostic tests directly within telehealth workflows, without leaving the care environment.
Once an order is placed, routing becomes a governed decision. Orders are automatically directed to approved lab partners based on geography, availability, service-level agreements, and clinical requirements. As a result, manual coordination is reduced, and diagnostic workflows remain predictable at scale.
2. Sample Collection & Logistics Coordination
After an order is confirmed, execution shifts from clinical to operational workflows. The platform manages how samples are collected, whether through home collection, partner lab visits, or kiosk-based sampling.
At this stage, visibility is critical. Status tracking keeps patients and providers informed, while delays or missed collections trigger follow-ups. This prevents drop-offs during one of the most fragile points in the diagnostic journey.
3. Result Ingestion & Data Normalization
Once samples are processed, results must return in a usable form. Lab results are ingested as structured data and normalized across providers to ensure consistency.
This normalization step is what makes diagnostics actionable. It supports analytics, trend analysis, and clean integration into EHRs and downstream clinical workflows, rather than leaving results trapped in static reports.
4. Clinical Review & Decision Support
With results available, responsibility returns to clinical decision-making. Findings are presented within clinician dashboards, with abnormal values prioritized for timely review.
At the same time, human oversight remains intact. Escalation rules guide follow-up actions while preserving clinician judgment, accountability, and traceability across the care episode.
5. Governance, Consent, & Audit Controls
Across every step, governance must be continuously enforced. This layer applies role-based access, manages patient consent, and maintains complete audit trails.
Every diagnostic action becomes traceable. This supports regulatory compliance, audit readiness, and enterprise risk management without relying on manual checks or retrospective validation.
Taken together, these components transform diagnostics into an integrated operational layer. They ensure virtual lab workflows remain reliable, compliant, and scalable as telehealth platforms grow.
Core Use Cases For Virtual Lab Integration In Telehealth
Virtual lab integration delivers the most value where diagnostics directly determine care continuity. For enterprises, these use cases show how telehealth evolves from isolated virtual visits into governed, outcome-driven care delivery.
1. Primary Care And Preventive Screening
Preventive care depends on early and repeatable diagnostics. However, when labs sit outside telehealth workflows, screening programs often lose momentum after the initial consultation, and follow-ups become inconsistent.
When diagnostics are embedded into telehealth workflows, continuity improves. Clinicians can order routine screenings during virtual visits and act on results without delay. As a result, screening completion rates rise and time to intervention shortens.
Real-world example:
Kaiser Permanente integrates lab ordering and result review across its virtual primary care programs. Preventive screenings such as lipid panels and metabolic tests are ordered digitally and reviewed within the same care environment, supporting early detection at scale.
With preventive workflows stabilized, enterprises can extend telehealth into long-term condition management.
2. Chronic Condition Management
Chronic conditions require continuous diagnostic insight rather than episodic testing. Conditions such as diabetes and thyroid disorders depend on recurring lab results to guide treatment adjustments over time.
Integrated virtual labs enable structured, repeatable monitoring. Scheduled testing aligns directly with care plans, allowing clinicians to track trends remotely and intervene earlier. This reduces unnecessary in-person visits while preserving clinical oversight.
Real-world example:
The National Health Service supports remote diabetes management programs where HbA1c testing is coordinated alongside virtual reviews. Lab results feed into longitudinal care records, enabling clinicians to manage large patient populations with consistent oversight.
As diagnostics become longitudinal, telehealth can support more specialized care programs.
3. Women’s Health And Hormonal Care
Hormonal care depends on precise and recurring diagnostic evidence. Fertility treatment, PCOS management, and menopause care all require regular lab assessments to tailor treatment safely and effectively.
Virtual lab integration allows hormonal testing to operate within ongoing telehealth programs. This ensures continuity across cycles of care without repeated clinic visits, improving patient engagement and clinical precision.
Real-world example:
Hims & Hers Health integrates diagnostic testing into its women’s health and hormonal care offerings. Lab results support clinician-led treatment decisions within structured virtual care workflows.
Beyond ongoing care, diagnostics also play a critical role during care transitions.
4. Post-Discharge And Transitional Care
The post-discharge window is one of the highest-risk phases of care. Lab monitoring is often required to detect complications early, yet coordination frequently breaks down once patients leave the hospital.
When virtual labs are integrated, diagnostic oversight continues beyond discharge. Abnormal results trigger timely follow-up, reducing preventable escalation and readmissions.
Real-world example:
Mayo Clinic incorporates remote monitoring and lab follow-up into post-discharge care pathways. Diagnostic data support early intervention across select service lines.
Taken together, these use cases show how diagnostics anchor continuity across telehealth care models.
By integrating virtual labs, enterprises transform telehealth into a scalable, accountable, and outcome-driven care system.
Enterprise Architecture For Virtual Lab Integration Into Telehealth Systems
Virtual lab integration at enterprise scale requires more than point integrations. Instead, it demands a layered architecture that governs diagnostics as part of the telehealth operating system.
Each architectural layer plays a distinct role. Together, they must support clinical continuity, partner variability, regulatory enforcement, and operational reliability without compromising scale.
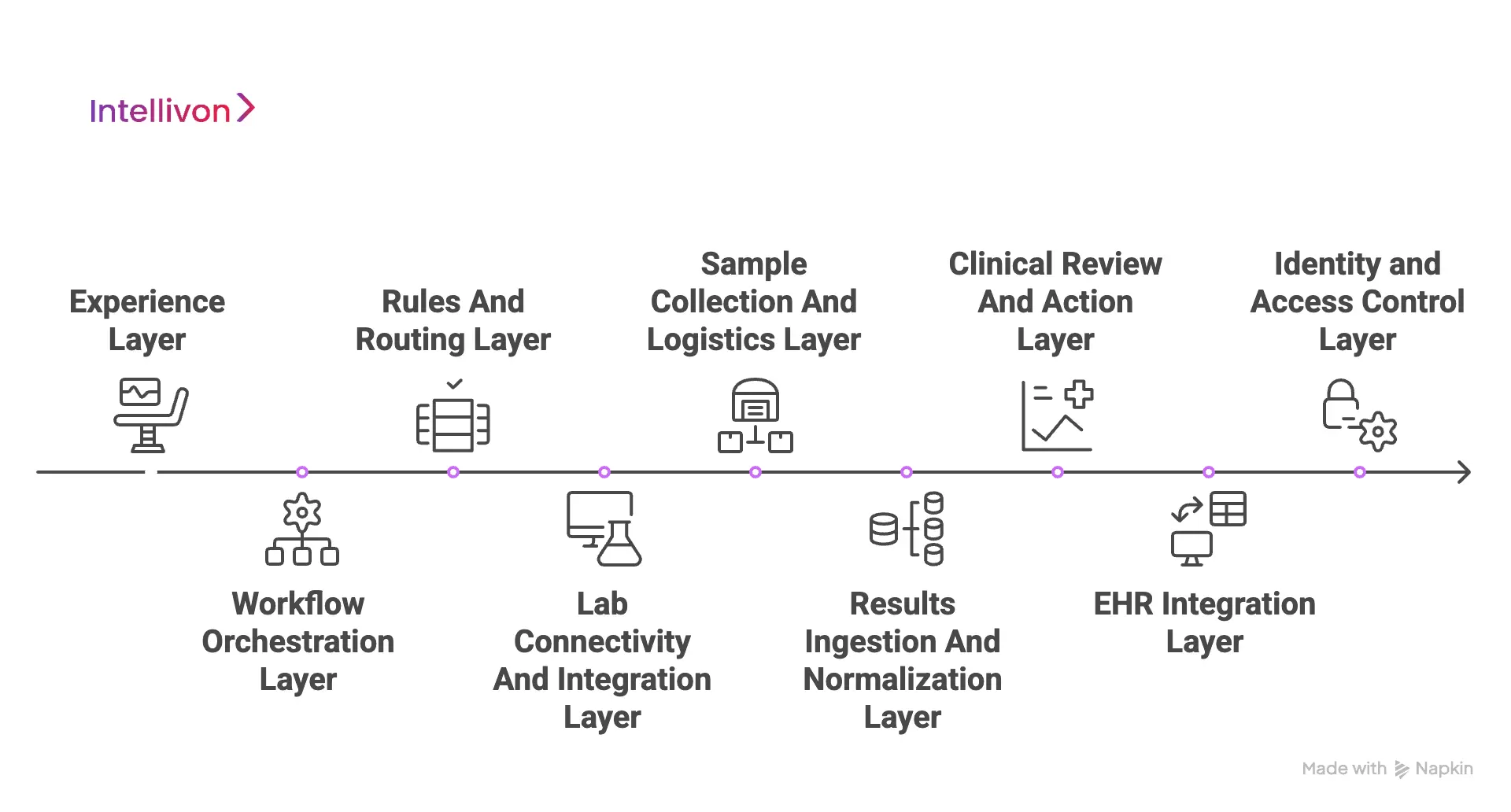
1. Experience Layer
Every diagnostic journey begins and ends at the experience layer. This includes patient-facing applications and clinician workspaces where lab actions originate and conclude.
Lab ordering, status tracking, and result review must feel native to the telehealth experience. Clear instructions, consent prompts, and next steps reduce friction and improve completion rates. For clinicians, results must appear in context and be aligned with the care episode. As a result, this layer sets expectations and drives adoption.
Once the experience is defined, workflows must progress predictably behind the scenes.
2. Workflow Orchestration Layer
The orchestration layer governs how diagnostics move from order to outcome. It manages sequencing, dependencies, reminders, and exception handling across all lab-related tasks.
Rather than relying on manual follow-ups, workflows advance automatically based on events and predefined rules. This ensures consistency across care journeys, even as volume grows. Without orchestration, diagnostic workflows fragment quickly.
To orchestrate effectively, the system must also decide where orders are routed.
3. Rules And Routing Layer
This layer determines how lab orders are routed across approved partners. Routing logic considers geography, test availability, turnaround time, cost, and contractual SLAs.
Clinical constraints and policy rules ensure orders meet regulatory and safety requirements. As partners change, routing rules can be updated without disrupting upstream workflows. This preserves flexibility while maintaining enterprise control.
With routing established, secure connectivity becomes the next requirement.
4. Lab Connectivity And Integration Layer
The connectivity layer manages secure interfaces with external laboratories. It supports standards such as FHIR and HL7 alongside partner-specific APIs.
Adapters normalize communication patterns and handle retries, failures, and acknowledgements. By isolating partner complexity from clinical workflows, integrations remain resilient and replaceable.
Once connectivity is established, execution shifts to sample collection.
5. Sample Collection And Logistics Layer
This layer coordinates how and where samples are collected. It supports home collection scheduling, partner lab visits, or kiosk-based sampling.
Status updates flow back into the platform to maintain visibility for both patients and clinicians. Delays or missed collections trigger automated follow-ups, reducing drop-offs during the most fragile stage of the diagnostic journey.
After samples are processed, results must be returned in a usable form.
6. Results Ingestion And Normalization Layer
Once results arrive, they are ingested as structured data rather than static documents. This layer standardizes units, reference ranges, and test identifiers across providers.
Normalization enables comparison, trend analysis, and downstream automation. Without this layer, results remain informational rather than actionable, limiting their clinical value.
With clean data available, responsibility returns to clinical decision-making.
7. Clinical Review And Action Layer
This layer supports how clinicians interpret and act on lab results. Abnormal findings are prioritized, while normal results follow predefined pathways.
Escalation rules guide follow-ups, treatment changes, or referrals without removing clinician judgment. Tasks and decisions are recorded within the care context, keeping accountability clear at every step.
To preserve continuity beyond a single encounter, data must persist longitudinally.
8. EHR Integration Layer
Diagnostic data must flow into the patient’s longitudinal record. This layer handles write-backs to EHRs and enterprise data stores.
Results, decisions, and actions are preserved across encounters. Integration ensures lab data informs future care rather than remaining siloed, enabling enterprise-wide visibility.
Across all layers, access and accountability must remain tightly controlled.
9. Identity And Access Control Layer
This layer enforces who can order, view, and act on diagnostic data. Role-based access and consent policies are applied consistently across workflows.
Delegation, revocation, and patient permissions are managed dynamically. Every access is traceable and policy-driven, protecting patient trust and regulatory posture.
When designed as a layered infrastructure, virtual lab integration strengthens telehealth rather than complicating it.
Each layer reinforces continuity, accountability, and scale, enabling enterprises to deliver diagnostics as part of a closed-loop care system.
How Virtual Lab Workflows Fit Into Telehealth Care Journeys
Virtual lab workflows only deliver value when they align with how care unfolds over time. In enterprise telehealth, diagnostics must support continuity, accountability, and timely action at every stage of the care journey. To understand this alignment, it helps to look at where diagnostics enter the journey and how they drive progress.
1. Pre-Consultation Diagnostics
In certain care scenarios, diagnostics are required before clinical decisions can begin. Pre-consultation labs allow evidence to guide the first interaction rather than postponing action until after the visit.
When virtual labs are integrated early, care starts with context. Tests are ordered in advance, and results are available at the time of consultation. As a result, decision cycles shorten, and clinical confidence improves from the outset.
Once care begins with evidence, diagnostics must continue to integrate seamlessly during the visit itself.
2. In-Consultation Lab Ordering
During virtual visits, clinicians often identify the need for diagnostic confirmation. If lab ordering occurs outside the consult workflow, fragmentation quickly follows.
Integrated platforms keep diagnostic actions inside the encounter. Clinicians place orders, capture consent, and initiate collection without leaving the visit. As a result, care moves forward without manual handoffs or lost follow-ups.
However, many diagnostic decisions still depend on what happens after the visit ends.
3. Post-Consultation Follow-Up
A significant portion of diagnostic-driven care occurs after the consultation. Without integrated workflows, results can stall, and actions may be delayed.
Virtual lab integration ensures results return to governed queues. Follow-ups, treatment changes, or escalations are triggered automatically, keeping accountability intact and care moving forward.
Beyond individual episodes, diagnostics must also support care over time.
4. Longitudinal Monitoring And Ongoing Care
Chronic and preventive care depend on repeated diagnostics rather than one-time testing. Virtual labs enable scheduled testing tied directly to care plans.
As results accumulate, patterns become visible. Trend analysis guides timely adjustments without requiring unnecessary visits, supporting continuous care while controlling operational load.
Even with structured workflows, exceptions remain inevitable.
5. Exception Handling And Escalation
Not every diagnostic journey follows a straight path. Missed collections, delayed results, or abnormal findings require immediate attention.
Integrated workflows surface exceptions early and route them to the appropriate clinical teams. This reduces risk, preserves accountability, and prevents small issues from escalating into costly failures.
When diagnostics are woven into every stage of the care journey, telehealth moves beyond episodic access.
It becomes a continuous, accountable care delivery system built to scale.
Compliance & Regulatory Considerations Of This Integration
Virtual lab integration introduces regulated diagnostic data directly into telehealth workflows. As a result, compliance requirements actively shape how platforms manage access, accountability, and data movement rather than sitting in the background.
For enterprises, this means compliance must be enforced through system design, not documentation. The following considerations show how regulation influences virtual lab workflows at every stage.
1. Data Protection And Consent
Data protection laws define how diagnostic data can be collected, accessed, and shared. In the US, HIPAA governs diagnostic data handling, while GDPR and the UK Data Protection Act impose strict rules around consent, purpose limitation, and data minimization.
As a result, consent must be captured at the moment of lab ordering and enforced continuously. Access controls, retention policies, and consent withdrawal must operate automatically as workflows progress, ensuring compliance without manual intervention.
Beyond data protection, regulators also expect clear ownership of diagnostic decisions.
2. Clinical Accountability And Result Ownership
Regulatory bodies require diagnostic responsibility to remain clearly assigned. In the UK, the General Medical Council mandates traceable clinical decision-making, while US state medical boards impose similar expectations for ordering and acting on tests.
Virtual lab workflows must preserve clinician attribution throughout the diagnostic journey. Platforms should clearly show who ordered a test, who reviewed the result, and how follow-up decisions were made, keeping accountability intact.
However, accountability also extends to the laboratories involved.
3. Lab Accreditation And Diagnostic Oversight
Telehealth platforms remain accountable for the labs they integrate with. In the US, laboratories must comply with CLIA standards, often supplemented by CAP accreditation. In the UK, diagnostic labs are regulated through UKAS accreditation and NHS-aligned standards.
As a result, partner governance cannot rely on contracts alone. Routing rules and onboarding controls must enforce accreditation requirements operationally, ensuring only approved labs participate in diagnostic workflows.
As telehealth expands geographically, data movement introduces additional complexity.
4. Cross-Border Data Handling And Storage
When diagnostic data crosses borders, regulatory risk increases significantly. GDPR restricts international data transfers, while HIPAA requires safeguards regardless of where data is processed or stored.
Therefore, platform architecture must support regional data routing and storage boundaries. Selective partner access and jurisdiction-aware controls are essential, without which scaling telehealth across markets becomes legally fragile.
Even with strong controls in place, compliance must remain provable.
5. Auditability And Incident Readiness
Audits focus on evidence, not intent. Both HIPAA and GDPR require detailed audit trails, while the NHS Data Security and Protection Toolkit emphasizes operational readiness and incident response.
Platforms must log every diagnostic event end-to-end. From order creation to clinical action, monitoring and response workflows ensure issues are detected early and resolved quickly.
When regulatory requirements are embedded directly into virtual lab workflows, compliance becomes predictable and enforceable. This allows telehealth platforms to scale diagnostics confidently across services, partners, and regions.
Role Of AI In Virtual Lab-Enabled Telehealth Platforms
AI plays a supporting role in virtual lab-enabled telehealth platforms. Its value lies in strengthening workflows, improving prioritization, and reducing operational friction, while clinical accountability remains with human decision-makers.
1. Intelligent Lab Ordering Support
AI assists clinicians at the point of ordering by surfacing relevant diagnostic suggestions based on symptoms, history, and care context. This reduces unnecessary testing and supports more consistent ordering practices.
Importantly, AI does not place orders independently. Clinicians retain full control, ensuring decisions remain explainable and compliant.
2. Result Prioritization And Risk Flagging
As lab results return, AI helps prioritize what requires immediate attention. Abnormal values, rapid changes, or risky trends are surfaced earlier, allowing clinicians to focus where intervention matters most.
This improves response times without automating clinical judgment. AI highlights risk, while clinicians decide action.
3. Longitudinal Trend Analysis
In ongoing care programs, AI analyzes lab trends over time rather than isolated results. This supports earlier detection of deterioration or improvement across chronic and preventive care journeys.
Trend insights help clinicians adjust care plans proactively, while maintaining transparency around how conclusions are reached.
4. Operational Optimization And Exception Detection
AI also supports the operational side of virtual labs. It identifies delayed collections, missing results, and workflow bottlenecks that could disrupt care continuity.
By flagging exceptions early, platforms reduce drop-offs and prevent small delays from becoming clinical or financial risks.
5. Governance And Human Oversight
AI usage must operate within defined boundaries. Under these platforms, explainability, audit trails, and human-in-the-loop controls ensure recommendations are traceable and reviewable.
When governed correctly, AI enhances scale and reliability without compromising trust, safety, or regulatory posture.
In virtual lab-enabled telehealth platforms, AI strengthens execution rather than decision ownership. Used responsibly, it improves efficiency, consistency, and outcomes while preserving clinical control.
How We Integrate Virtual Labs into Enterprise Telehealth Systems
At Intellivon, we approach virtual lab integration as a core platform capability, and not a technical add-on. In the platforms we build, diagnostics influence how care is delivered, governed, and completed, which means integration decisions affect clinical accountability, regulatory exposure, and long-term scalability.
Our methodology is built for healthcare enterprises operating across regions, service lines, and partner ecosystems. Additionally, we focus on designing systems that remain stable as volumes grow, partners change, and regulations evolve. At the same time, each step in our process strengthens continuity, predictability, and control across the diagnostic journey.
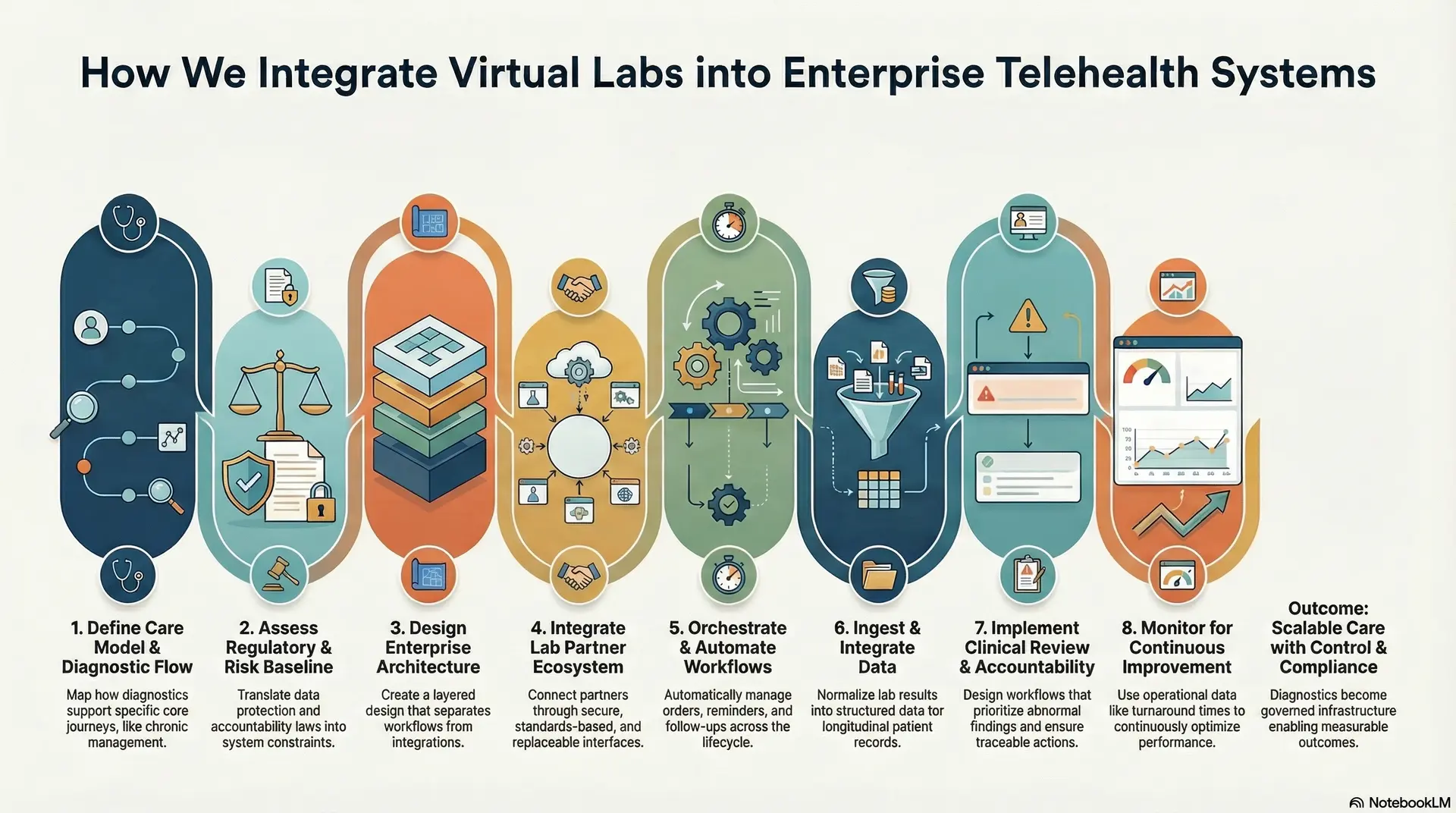
1. Care Model And Diagnostic Flow Definition
We begin by mapping how diagnostics support real-world care delivery. This includes identifying when labs are required, how frequently they recur, and how results influence downstream clinical decisions.
We align diagnostic flows with specific care models such as preventive care, chronic management, or post-discharge monitoring. This ensures labs reinforce the care journey rather than disrupt it. By defining these flows early, we avoid fragmented workflows and unclear ownership later.
2. Regulatory And Risk Baseline Assessment
Next, we assess the regulatory landscape governing diagnostic workflows. This includes data protection laws, clinician accountability requirements, and lab accreditation obligations across all operating regions.
We translate these requirements into system-level constraints. Consent capture, access controls, and audit expectations are defined upfront. As a result, compliance is enforced automatically through platform behavior rather than manual oversight.
3. Enterprise Architecture And Layer Design
We design a layered architecture that separates clinical workflows from integrations and governance. Each layer has clear responsibilities and well-defined boundaries.
This approach allows enterprises to scale diagnostics without rebuilding workflows. It also isolates partner complexity, ensuring lab changes do not ripple through clinician-facing systems. The architecture remains flexible, resilient, and maintainable over time.
4. Lab Partner And Ecosystem Integration
Approved lab partners are integrated through secure, standards-based interfaces. Routing logic accounts for geography, test availability, turnaround times, and contractual service levels.
We design integrations to be replaceable rather than hard-coded. This allows enterprises to expand or modify lab networks without disrupting care delivery. Partner governance remains centralized and enforceable.
5. Workflow Orchestration And Automation
We implement orchestration logic that governs how lab orders move from placement to completion. Dependencies, reminders, and follow-ups are managed automatically across the diagnostic lifecycle.
This reduces reliance on manual coordination and minimizes missed steps. Exceptions such as delayed collections or incomplete results are surfaced early, preserving care continuity and operational efficiency.
6. Data Ingestion And Record Integration
Lab results are ingested as structured data and normalized across providers. Units, reference ranges, and identifiers are standardized to ensure consistency and accuracy.
Normalized data flows into longitudinal patient records and enterprise systems. This supports trend analysis, audit readiness, and informed decision-making across future encounters. Diagnostics become computable, not just viewable.
7. Clinical Review And Accountability Controls
We design workflows that present results in a clinical context. Abnormal findings are prioritized, and escalation paths are clearly defined based on care protocols.
Clinicians retain full control over decisions. Every review and action is traceable, preserving accountability across the care episode. This strengthens both governance and clinician trust.
8. Monitoring And Continuous Improvement
After deployment, we monitor key operational signals such as turnaround times, drop-offs, and result-to-action latency. These insights highlight where workflows need refinement.
We use this feedback to continuously optimize performance. As programs scale, the system adapts without compromising reliability, compliance, or care quality.
Virtual lab integration succeeds when diagnostics are treated as governed infrastructure, enabling enterprise telehealth platforms to scale care delivery with control, compliance, and measurable outcomes.
Interoperability Challenges And How To Solve Them
Interoperability is one of the most underestimated risks in virtual lab–enabled telehealth. At enterprise scale, failures rarely come from missing APIs. They emerge from mismatched standards, broken workflows, and unclear accountability across systems.
At Intellivon, we address interoperability as a platform problem, and not an integration task. Our approach is shaped by how systems behave under real clinical, regulatory, and operational pressure.
1. Fragmented Data Standards
Labs often return results in different formats, units, and reference ranges. This is because even when standards exist, implementation varies widely across providers.
Intellivon solves this through a canonical data model and normalization layer. Here, the results are standardized at ingestion, allowing downstream workflows, analytics, and clinical decisions to remain consistent regardless of the source.
2. Inconsistent HL7 / FHIR Implementations
Many labs and EHRs claim FHIR or HL7 support, but implement only subsets. This leads to brittle integrations and silent data loss. We design adapter layers that absorb partner variability.
Here, core workflows remain stable, while partner-specific differences are handled in isolation, reducing long-term integration risk.
3. Legacy EHR Constraints And Vendor Lock-In
Enterprise health systems often operate legacy EHRs with limited interoperability. Within these platforms, direct integration can slow innovation and restrict workflow design.
Intellivon decouples lab workflows from EHR limitations. Additionally, diagnostic data flows through an independent orchestration layer, with controlled write-back to EHRs, preserving continuity without sacrificing flexibility.
4. Asynchronous Workflows And Timing Mismatches
Lab workflows are inherently asynchronous. Orders, collections, and results do not align neatly with real-time telehealth interactions.
We use event-driven orchestration to manage timing gaps. Workflows advance based on system events, not assumptions, ensuring follow-ups and escalations occur reliably.
5. Inconsistent Patient And Test Identifiers
Different systems often use different identifiers for patients, tests, and encounters. This creates reconciliation errors and audit challenges.
Intellivon applies identity resolution and mapping at the platform level. Identifiers are unified early, allowing traceability to remain intact across systems and partners.
6. Accountability Gaps Across Integrated Systems
When systems interoperate poorly, responsibility becomes unclear. Orders, results, and actions lose ownership across handoffs.
We design workflows that preserve attribution end-to-end. Every diagnostic event is linked to a clinician, a decision, and a timestamp, supporting both governance and trust.
Interoperability succeeds when platforms are designed for variability, not ideal conditions. By solving for real failure modes, Intellivon enables enterprises to integrate virtual labs without sacrificing control, compliance, or scale.
Examples Of Telehealth Platforms That Support Virtual Lab Integrations
Several telehealth platforms have moved beyond basic referrals to embed diagnostics directly into their operating models. These examples show how virtual lab integration works in practice when platforms are designed to support continuity, accountability, and scale.
1. DrChrono
DrChrono operates as a full-stack telehealth and EHR platform built for clinical efficiency. This platform combines HIPAA-compliant video visits, charting, prescribing, and billing within a single system.
Virtual lab integration is embedded directly into clinician workflows. At the same time, providers place electronic lab orders to partners such as Labcorp and Quest from within the EHR. After this, results flow back automatically into patient charts, allowing clinicians to review findings and act without leaving the platform.
2. DocVilla
DocVilla is a telemedicine-first EHR designed to reduce operational fragmentation. This platform supports virtual visits, documentation, prescriptions, and care coordination in one interface.
Lab integration replaces fax-based and portal-driven processes with direct electronic connectivity. Here, clinicians order tests and retrieve results from integrated partners like AccuReference within DocVilla workflows. This improves turnaround times and keeps diagnostic follow-ups structured and traceable.
3. Meditab
Meditab provides an all-in-one EHR and telehealth solution aimed at simplifying clinical operations. In their workflows, telemedicine visits, clinical notes, prescriptions, and diagnostics are managed in a unified system.
Virtual labs are integrated through built-in diagnostic ordering modules. Here, clinicians can request lab tests during virtual visits and review results alongside clinical documentation. This enables seamless remote care without relying on external systems.
4. Hurdle
Hurdle focuses on powering remote diagnostics for telehealth and digital health brands. It operates as a white-label platform that supports clinician-led and patient-initiated testing.
Virtual lab workflows are managed end-to-end. Under this system, tests are requested through dashboards, processed by UKAS-accredited labs, and results are delivered through secure portals. This allows telehealth providers to offer regulated diagnostic services without building lab infrastructure themselves.
5. GetLabTest.com
GetLabTest.com combines telehealth consultations with AI-assisted diagnostic interpretation. The platform allows users to order lab tests alongside virtual doctor reviews.
Lab results are analyzed using AI models trained on large clinical datasets. Findings are presented through intuitive dashboards for clinician review, supporting faster interpretation while keeping medical decisions clinician-led. This approach integrates diagnostics, AI, and telehealth into a single workflow.
These platforms demonstrate that virtual lab integration works best when diagnostics are embedded into telehealth workflows rather than handled externally. At enterprise scale, this approach improves continuity, accountability, and the ability to deliver closed-loop care.
Conclusion
Telehealth platforms deliver value only when care journeys reach completion. Within these platforms, virtual labs play a critical role in closing the gap between consultation and outcome by embedding diagnostics directly into care workflows. When testing, results, and follow-ups operate inside the platform, enterprises gain clearer accountability, stronger clinical continuity, and predictable operational performance.
Enterprises that integrate diagnostics move beyond episodic virtual visits. Through this, they gain control over care progression, reduce avoidable escalations, and align clinical delivery with reimbursement and compliance requirements. As telehealth continues to expand across populations and service lines, platform-led care models will define sustainable growth. In this future, diagnostics are not optional. They are foundational infrastructure.
Build A Virtual Lab-Enabled Telehealth Platform With Intellivon
At Intellivon, we build virtual lab-enabled telehealth platforms as enterprise operating systems, not virtual care tools stitched together with external diagnostics. Our platforms are designed to govern how diagnostics are ordered, how results flow into clinical decision-making, and how accountability and compliance are preserved as care scales across service lines.
Each solution is engineered for healthcare organizations operating at scale. Platforms are architecture-first and compliance-led, with diagnostic workflows embedded across care journeys, identity controls, data governance, and audit layers. As programs expand across populations, lab partners, and regions, clinical oversight, data integrity, and operational predictability remain intact.
Why Partner With Intellivon?
- Enterprise-grade platform architecture aligned with diagnostic-driven care delivery and scalable telehealth operations
- Deep interoperability expertise across EHRs, lab networks, identity frameworks, analytics platforms, and enterprise infrastructure
- Compliance-by-design delivery supporting HIPAA, GDPR, clinical accountability, lab accreditation, and audit readiness
- AI-assisted orchestration that improves diagnostic follow-through and operational efficiency without removing clinician control
- Proven enterprise delivery model with phased rollout, workflow validation, and long-term platform scalability
Talk to Intellivon’s healthcare platform architects to explore how a virtual lab-enabled telehealth platform can integrate into your existing ecosystem, reduce operational friction, and deliver closed-loop, accountable care at scale.
FAQs
Q1. What is virtual lab integration in telehealth?
A1. Virtual lab integration embeds diagnostic ordering, sample collection, result delivery, and follow-up directly into telehealth workflows. It allows clinicians to move from consultation to evidence-based action without relying on external portals or manual coordination.
Q2. How do virtual labs improve telehealth outcomes?
A2. Virtual labs close care gaps by ensuring diagnostics are completed and acted upon. This reduces missed follow-ups, repeat consultations, and unnecessary in-person escalation while improving care completion and accountability.
Q3. Is virtual lab integration compliant with healthcare regulations?
A3. Yes, when designed correctly. Enterprise platforms enforce HIPAA, GDPR, UK data protection, lab accreditation, and clinical accountability through system behavior, not manual processes or policy workarounds.
Q4. Can virtual labs be integrated with existing EHR systems?
A4. Virtual labs can integrate with existing EHRs using standards such as FHIR and HL7. Here, results are normalized and written back into longitudinal patient records while keeping workflows decoupled from EHR limitations.
Q5. What does it cost to add virtual lab integration to a telehealth platform?
A5. Costs vary based on care models, compliance requirements, lab partners, and scale. This is because enterprise platforms focus on long-term ROI by reducing operational leakage, improving care completion, and supporting scalable diagnostic workflows.