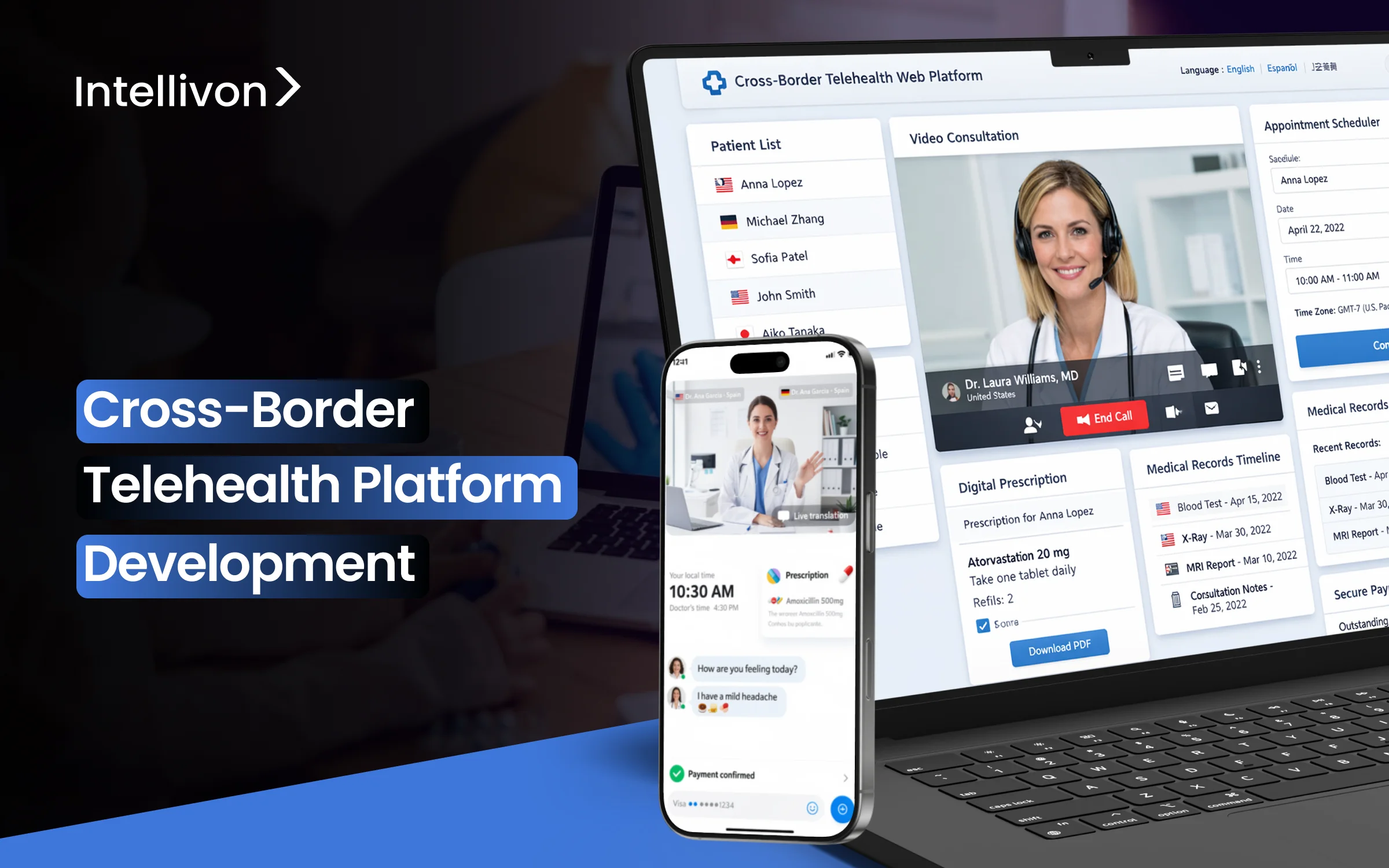
Telehealth platforms usually manage thousands of consultations each day within an area. When a patient in another country requests a consultation with a specialist in another country, the real roadblocks arrive. Suddenly, operation teams find themselves dealing with conflicting consent requirements, medical licensing issues, and data residency rules that don’t match. What should be a routine appointment turns into a compliance review that takes days.
Healthcare companies expanding internationally face this challenge all the time. Telehealth adoption continues to grow across borders. However, most platforms were designed for single-jurisdiction operations, and not for the complex regulations and processes involved in serving patients in multiple countries.
At Intellivon, we have created cross-border telehealth platforms for companies facing this exact issue. One problem often arises, which is managing real-time consent across areas where patient consent rules vary greatly. To counter this, we developed a dynamic consent engine that automatically adjusts to local regulations. This cuts out manual compliance checks and reduces deployment times from months to weeks. This blog discusses how we build these platforms from the ground up.
Key Drivers Behind Cross-Border Telehealth Adoption
From an enterprise standpoint, cross-border telehealth sits where regulated care, global operations, and digital health infrastructure intersect. Unlike domestic telehealth, success depends on platforms that understand jurisdictional boundaries. Licensing, prescribing, data protection, and accountability rules must adjust automatically as care moves across borders.
Market signals reinforce this shift. The global cross-border telehealth platform market was valued at approximately USD 4.7 billion in 2024. Growth is driven by rising demand for international consultations, specialist second opinions, and remote follow-up care.
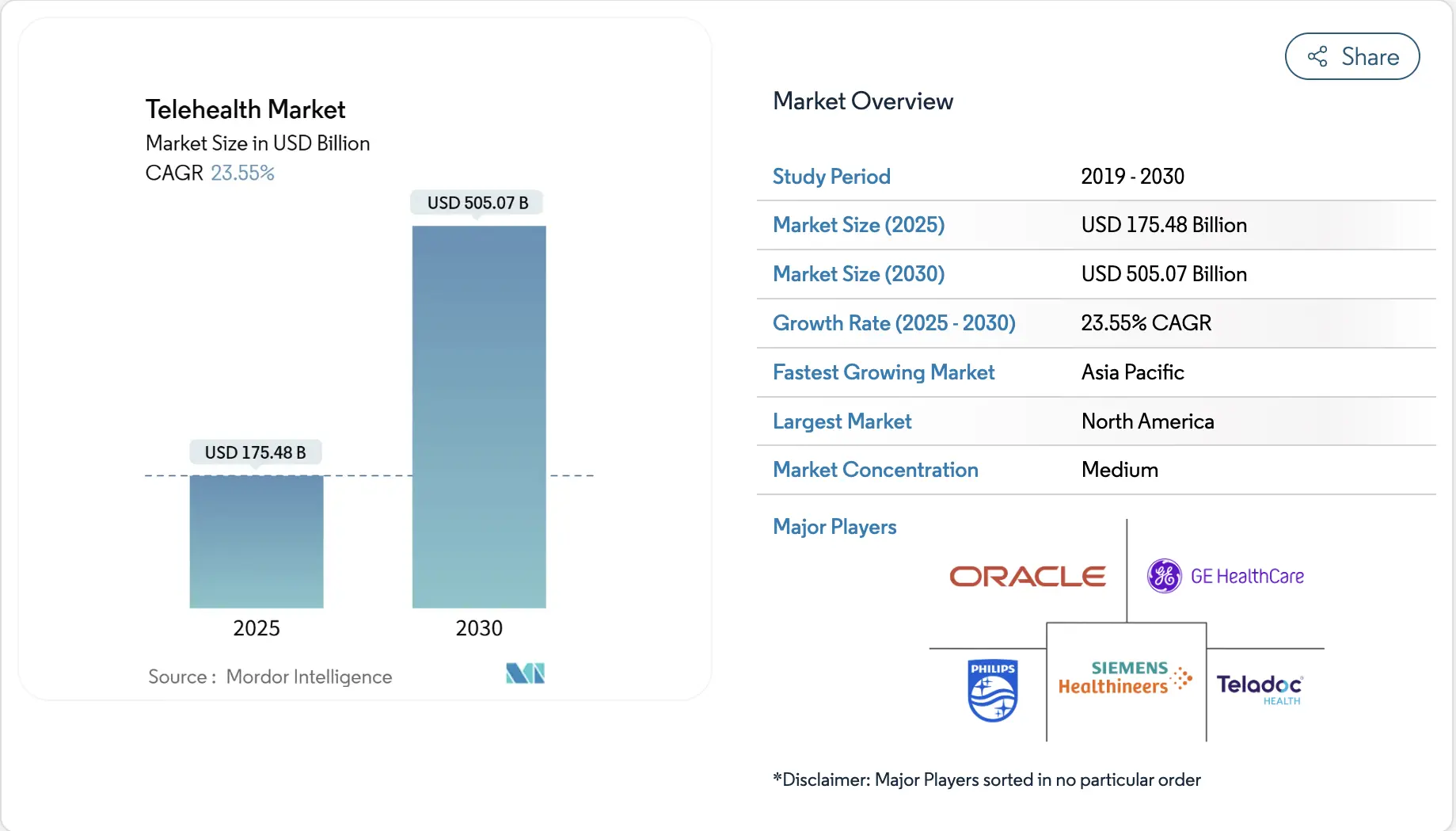
At the same time, the broader telehealth market is projected to reach approximately USD 175.5 billion in 2025 and is expected to grow to over USD 505 billion by 2030, reflecting sustained expansion at an annual growth rate above 23%. As telehealth scales globally, regulatory enforcement and accountability increasingly determine which platforms can operate across borders sustainably.
Key Growth Insights
Several structural forces are accelerating cross-border telehealth adoption. Together, they explain why international virtual care is shifting from isolated use cases to repeatable enterprise growth models.
- Care access gaps: In many regions, specialist availability remains uneven. As a result, patients increasingly seek international virtual consultations and second opinions when local options are limited or delayed.
- Economics of medical travel: At the same time, clearer pricing models and bundled digital services are making cross-border virtual care more attractive. Self-pay patients gain predictable costs without the burden of physical travel.
- Digital readiness: In parallel, broader broadband access, rising smartphone penetration, and AI-driven language support are improving remote consultation quality. Secure data exchange across borders has also become more reliable.
- Insurer-led adoption: In addition, global health plans are expanding telehealth networks to support travelers and expatriates. This enables earlier intervention and helps reduce avoidable downstream claims.
- Regulatory evolution: Finally, policy frameworks across Europe, the GCC, and parts of Asia are evolving. Simplified approaches to licensing, e-prescribing, and remote care delivery are lowering barriers to scale.
Cross-border telehealth platforms often follow a few proven operating models. For example, UK-based specialist networks provide paid second opinions and structured follow-up care to international patients. This creates incremental revenue without requiring physical expansion.
In both models, long-term success depends on enforcement at the platform level. Licensing, prescribing, and data controls must operate automatically. When these rules are embedded into system behavior, organizations can scale predictably while keeping compliance risk contained.
What Is a Cross-Border Telehealth Platform?
A cross-border telehealth platform enables regulated care delivery across national boundaries while preserving clinical accountability, licensing rules, and data protection requirements. Unlike domestic telemedicine, it must adapt workflows as care moves between jurisdictions, providers, and legal frameworks.
Successful platforms such as Babylon Health and Teladoc Health work because they embed compliance into system behavior. They manage clinician attribution, consent, and longitudinal records across regions. As a result, care continuity remains intact even when geography changes.
At scale, these platforms operate as governed healthcare infrastructure, not booking tools.
What Changes as Telehealth Systems Become Cross-Border
When telehealth moves beyond a single country, the operating model changes fundamentally. What works for domestic virtual care often breaks once licensing, data residency, and clinical accountability span multiple jurisdictions.
As a result, cross-border platforms must behave less like appointment tools and more like governed healthcare infrastructure. Below is a practical comparison that highlights where the shift happens.
| Area | Standard Telehealth Systems | Cross-Border Telehealth Platforms |
| Licensing | Assumes a single national licensing framework | Enforces clinician eligibility based on patient and provider jurisdiction |
| Prescribing | Follows one country’s prescribing rules | Applies region-specific prescribing and medication restrictions automatically |
| Data storage | Centralized data storage is common | Data residency and localization rules vary by country |
| Consent management | Static consent flows | Dynamic consent adjusted to local legal requirements |
| Care continuity | Often visit-based and episodic | Longitudinal records preserved across borders |
| Accountability | Clear within one legal system | Shared responsibility across jurisdictions |
| Risk management | Limited regulatory complexity | Continuous regulatory and audit oversight is required |
These differences change how platforms must be designed. Cross-border systems need jurisdiction awareness at every layer. Workflow logic, access control, and data handling must adjust automatically as care crosses borders.
In practice, scalability depends on governance, not volume. Platforms that embed these controls early can expand safely. Those that do not often stall once regulatory scrutiny increases.
Core Use Cases of Cross-Border Telehealth Platforms
Cross-border telehealth platforms enable regulated international care by preserving continuity, enforcing jurisdiction-aware workflows, and scaling enterprise healthcare without increasing compliance risk.
Cross-border telehealth platforms succeed when they solve repeatable enterprise problems. These use cases emerge from operational gaps, regulatory pressure, and the need to extend care without expanding physical footprint. Each use case below reflects how global healthcare organizations apply cross-border telehealth in practice.
1. International Specialist Second Opinions
Many enterprises struggle to provide timely access to niche specialists across regions. As a result, patients seek second opinions beyond their home country, often after diagnosis or treatment planning.
Cross-border telehealth enables structured specialist reviews without transferring care ownership. The original provider retains responsibility, while the specialist contributes expertise within defined boundaries. Therefore, accountability remains clear, and regulatory exposure stays controlled.
This model depends on platforms that preserve clinician attribution, consent, and full clinical context across jurisdictions. Without that continuity, second opinions introduce risk rather than clarity.
Post-Diagnosis Follow-Ups Across Borders
Care often fragments once patients travel or relocate. Follow-up visits, treatment adjustments, and recovery monitoring become difficult to coordinate across countries.
Cross-border telehealth allows the same clinician or care team to remain involved after the initial encounter. As a result, care plans stay consistent, and decisions remain grounded in prior context.
For enterprises, this reduces unnecessary handoffs and readmissions. However, success depends on enforcing jurisdiction-specific prescribing and documentation rules automatically.
Expatriate and Traveling Workforce Healthcare
Global employers and insurers face rising costs when employees seek emergency care abroad. Many cases escalate simply because timely guidance is unavailable.
These apps support early triage, virtual consultations, and care coordination for expatriates and travelers. This enables intervention before conditions worsen or require in-person escalation.
From an enterprise lens, the value lies in predictability. Platforms must route care based on location, eligibility, and local regulations without manual oversight.
Cross-Border Mental Health Programs
Mental health care benefits from continuity and trust. For international patients, switching providers due to relocation often disrupts progress.
Cross-border telehealth allows patients to continue therapy with the same clinician across regions. This is especially relevant for long-term treatment plans and employer-sponsored programs.
However, licensing and consent rules vary widely for mental health services. Platforms must enforce session eligibility and documentation standards by jurisdiction.
Global Chronic Care Monitoring and Support
Chronic conditions do not pause when patients move. Monitoring, education, and follow-ups must continue regardless of geography.
These telehealth apps support ongoing engagement through virtual check-ins and coordinated care workflows. Therefore, enterprises can maintain outcomes without duplicating teams in every region.
This use case requires tight integration between monitoring data, clinician workflows, and regional compliance controls. Without platform governance, risk grows quietly over time.
Insurer and Health System Virtual Care Networks
Some insurers and health systems deploy cross-border telehealth as part of broader virtual care networks. These networks support members across countries while preserving cost control.
Telehealth becomes the entry point for triage, follow-ups, and care navigation. As a result, unnecessary in-person visits decline, and utilization becomes more predictable.
Long-term success depends on embedding accountability into the platform. Manual oversight does not scale as networks expand.
Across all these use cases, growth follows governance. This is because cross-border telehealth platforms create value when they enforce rules automatically while preserving continuity across borders.
How Cross-Border Telehealth Platforms Work
Cross-border telehealth platforms deliver regulated international care through jurisdiction-aware workflows that preserve continuity, enforce compliance, and adapt automatically as care crosses borders.
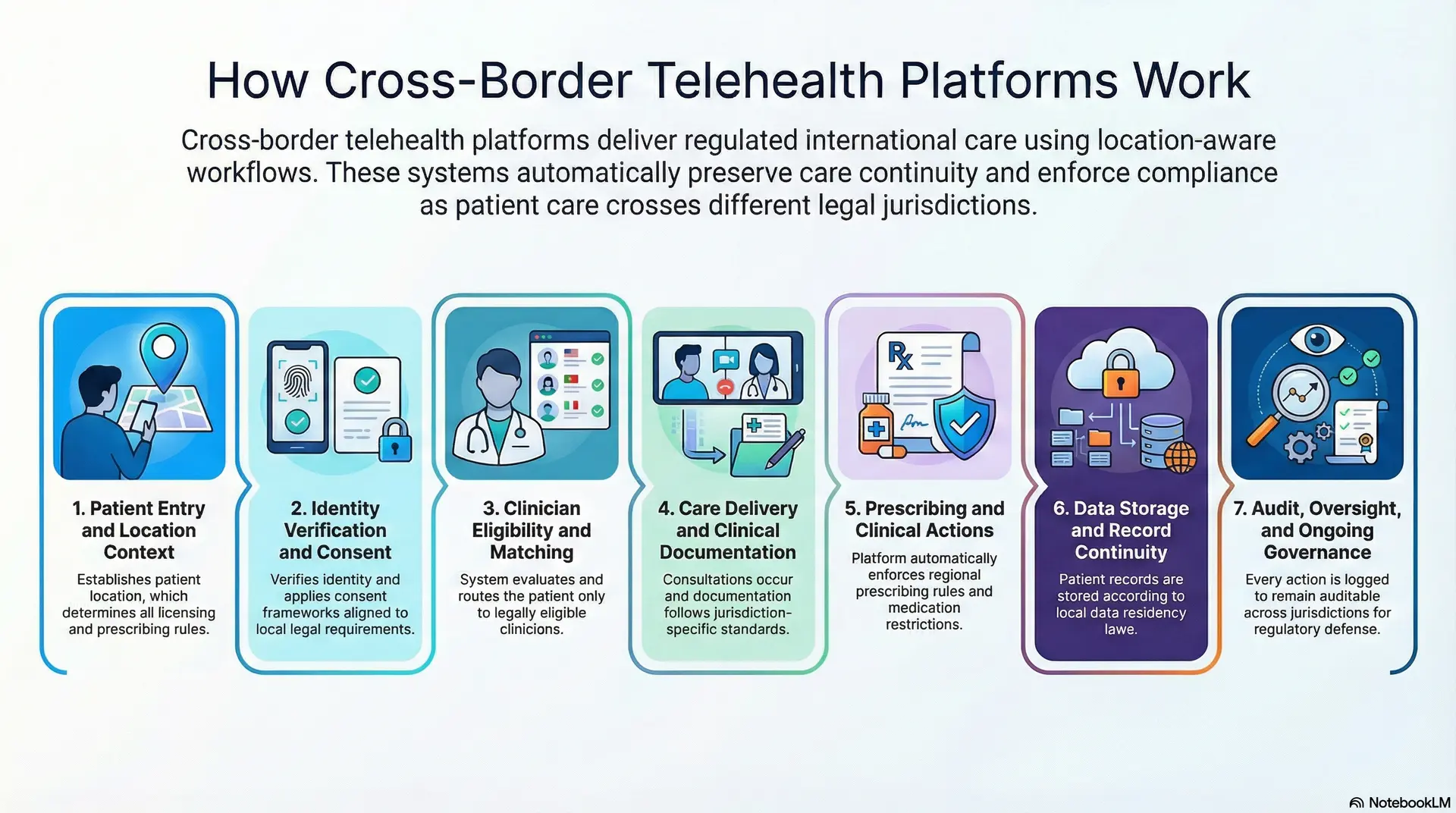
Step 1: Patient Entry and Location Context
Every workflow starts by establishing where the patient is located. Geography is not metadata. It determines licensing eligibility, prescribing rules, and data handling from the first interaction.
As a result, the platform captures location early and keeps it active throughout the care journey.
Step 2: Identity Verification and Consent
Once the location is known, identity and consent follow. The platform verifies patient identity and applies consent frameworks aligned to local legal requirements.
Consent is not static. It adjusts based on jurisdiction, care type, and data usage. This prevents downstream compliance gaps.
Step 3: Clinician Eligibility and Matching
The system evaluates which clinicians are legally allowed to provide care for that patient. Licensing, specialty scope, and regional rules guide routing decisions.
Only eligible providers appear in the workflow. This removes manual checks and reduces operational risk.
Step 4: Care Delivery and Clinical Documentation
Consultations occur through video, async messaging, or hybrid formats. During care delivery, documentation follows jurisdiction-specific standards.
Clinical notes, decisions, and escalations are recorded in a way that preserves accountability across borders.
Step 5: Prescribing and Clinical Actions
If treatment actions are required, the platform enforces regional prescribing rules automatically. Medication eligibility, restrictions, and follow-up requirements vary by country.
This ensures clinical intent translates into compliant execution.
Step 6: Data Storage and Record Continuity
Patient records are stored and accessed according to data residency requirements. At the same time, longitudinal history remains intact for authorized clinicians.
Continuity is preserved without violating localization laws.
Step 7: Audit, Oversight, and Ongoing Governance
Every action is logged. Access, decisions, and data flows remain auditable across jurisdictions.
This final layer enables enterprises to scale cross-border care while maintaining regulatory defensibility over time.
62% of Out-of-State Telemedicine Visits Follow Prior In-Person Care
Cross-border telehealth is often discussed as a virtual-first care model. However, real usage patterns tell a different story. Primary data shows that 62.6% of out-of-state telemedicine visits happened after a patient had already met the same clinician in person.
This detail changes how remote care should be evaluated. Regulators do not assess all telemedicine equally. When virtual care extends an existing clinical relationship, it carries more clinical context and clearer accountability. Therefore, continuity becomes a regulatory asset, not just a clinical preference.
This reality should directly shape how cross-border telehealth platforms are built.
1. Continuity Reduces Clinical and Legal Exposure
When a clinician already knows the patient, remote care operates as an extension of treatment. Here, the medical history is trusted, and the context is preserved. As a result, decisions around escalation, prescribing, and follow-up become more defensible.
From a regulatory lens, this continuity strengthens duty-of-care alignment. It also reduces ambiguity around responsibility and oversight. For enterprises, this lowers legal exposure without slowing care delivery or expanding operational burden.
2. Cross-Border Telehealth Often Starts After the First Visit
In practice, many successful cross-border programs do not begin with virtual consultations. Instead, they activate after diagnosis, care planning, or an initial in-person evaluation.
Telehealth then supports follow-ups, monitoring, and second opinions as patients move across regions. This sequence matters because it aligns closely with how regulators interpret clinical responsibility and jurisdictional oversight. Therefore, virtual care works best when it reinforces an existing care pathway.
3. Platform Design Must Preserve Relationship Context
To support continuity, platforms must retain clinician attribution and encounter history across borders. Identity resolution must remain intact. Longitudinal records must travel with the patient, not be fragmented by geography.
When this context breaks, continuity exists only on paper. Execution fails quietly, often without immediate visibility. This is where many cross-border platforms struggle as scale increases.
Cross-border telehealth scales safely when platforms protect continuity and attribution. At the same time, volume alone does not create sustainable care models.
Architecture of a Cross-Border Telehealth Platform
Cross-border telehealth architecture must run like regulated infrastructure, because geography changes what is allowed at every step.
Therefore, the platform needs clear layers that separate responsibility and reduce failure points. Below are eight enterprise-grade layers that support predictable scale.
1. Global Access and Identity Layer
This layer establishes who the user is and where they are located, because location drives eligibility and compliance. Multi-region authentication reduces friction while keeping entry conditions consistent across geographies.
Role-based access control limits what each role can view, change, and export, which protects PHI and reduces insider risk. Identity resolution matters when patients travel, relocate, or use different identifiers across systems, so the platform must link the record safely.
In addition, clinician identity must remain provable for audit and accountability. When this layer is weak, every downstream control becomes unreliable, including prescribing and documentation.
2. Care Delivery Layer
This layer delivers the actual consultation experience across video, chat, and asynchronous formats, based on what each region permits. Scheduling must be time-zone aware, because cross-border care fails quickly when time logic breaks.
The system should support bandwidth variability and device diversity, since user conditions differ by geography. In addition, the platform must capture structured encounter context during delivery, not after the call.
Clinical documentation hooks should be present inside the workflow, so notes and orders do not drift into side channels. When delivery is consistent and dependable, adoption rises without creating operational chaos.
3. Clinical Workflow and Case Management Layer
A visit is only one moment in the care journey, so this layer manages what happens before and after. It controls intake, triage, routing, follow-ups, referrals, and task ownership across teams. It also keeps escalation paths clear, so responsibility never becomes ambiguous across borders.
Structured templates reduce variation, documentation stays audit-ready, and is clinically useful. In addition, queueing and SLAs help teams operate at enterprise volumes without losing accountability. Without this layer, cross-border programs look fine in pilots, then break under real utilization.
4. Compliance and Governance Layer
This layer turns policy into system behavior, so teams do not rely on training alone. It enforces jurisdiction-specific licensing constraints, prescribing boundaries, consent requirements, and retention rules automatically. It also manages audit logs and traceability, so every access and decision can be reconstructed.
In addition, it supports policy versioning, because regulations evolve and enterprises must prove which rule was active when. Controls should apply consistently across interfaces, including admin tools and integrations. When governance is embedded, scale becomes safer, and leadership gains defensibility during audits.
5. Data and Interoperability Layer
Cross-border platforms need disciplined data movement because not all data can travel freely. This layer connects to EHRs and enterprise systems while respecting data residency and localization requirements. HL7 and FHIR exchange patterns help standardize clinical data, and handoffs become cleaner and less error-prone.
It should also support longitudinal records, so clinicians see the patient’s history across encounters and countries. In addition, consent must govern data sharing at the field and document level. When interoperability is designed well, continuity remains real, and duplication costs fall.
6. Security and Privacy Layer
Security must run continuously across regions because of the cross-border expansion of the attack surface. This layer covers encryption in transit and at rest, key management, secrets handling, and secure session controls. It also supports zero-trust enforcement, so access is verified every time, not assumed.
In addition, privacy controls such as tokenization or field-level protection reduce exposure for high-risk data elements. Threat detection and incident response hooks should integrate with enterprise SIEM workflows. When security is treated as a first-class layer, the platform earns trust from legal, compliance, and clinical leadership.
7. Intelligence and Orchestration Layer
Cross-border care requires smarter routing, because eligibility changes by region, specialty, and policy. This layer matches patients to clinicians who are legally permitted and operationally available. It also applies risk flags and escalation logic, so red flags surface early, and action becomes consistent.
In addition, orchestration coordinates tasks across services, so workflows do not fragment across tools. Automation should reduce manual checks while still keeping humans in control of clinical judgment. When orchestration is strong, teams scale without adding headcount at the same rate as volume.
8. Platform Infrastructure and Observability Layer
Enterprise cross-border deployments need resilience because regional outages and latency are inevitable. This layer handles multi-region hosting, traffic routing, failover, disaster recovery, and capacity scaling. Observability tracks logs, metrics, and traces, so performance issues are identified before they hit clinical operations. It also monitors access anomalies and workflow failures, and risks are caught early.
In addition, SLOs and alerting create operational discipline, which supports regulated delivery. When infrastructure and observability are mature, cross-border growth becomes predictable rather than fragile.
Regulatory & Compliance Challenges in Cross-Border Telehealth
Cross-border telehealth introduces regulatory complexity that does not exist in domestic care. Rules change by geography, and responsibility becomes harder to define as patients, clinicians, and data move across jurisdictions.
Below are the most common challenges enterprises face and how they are addressed at the platform level.
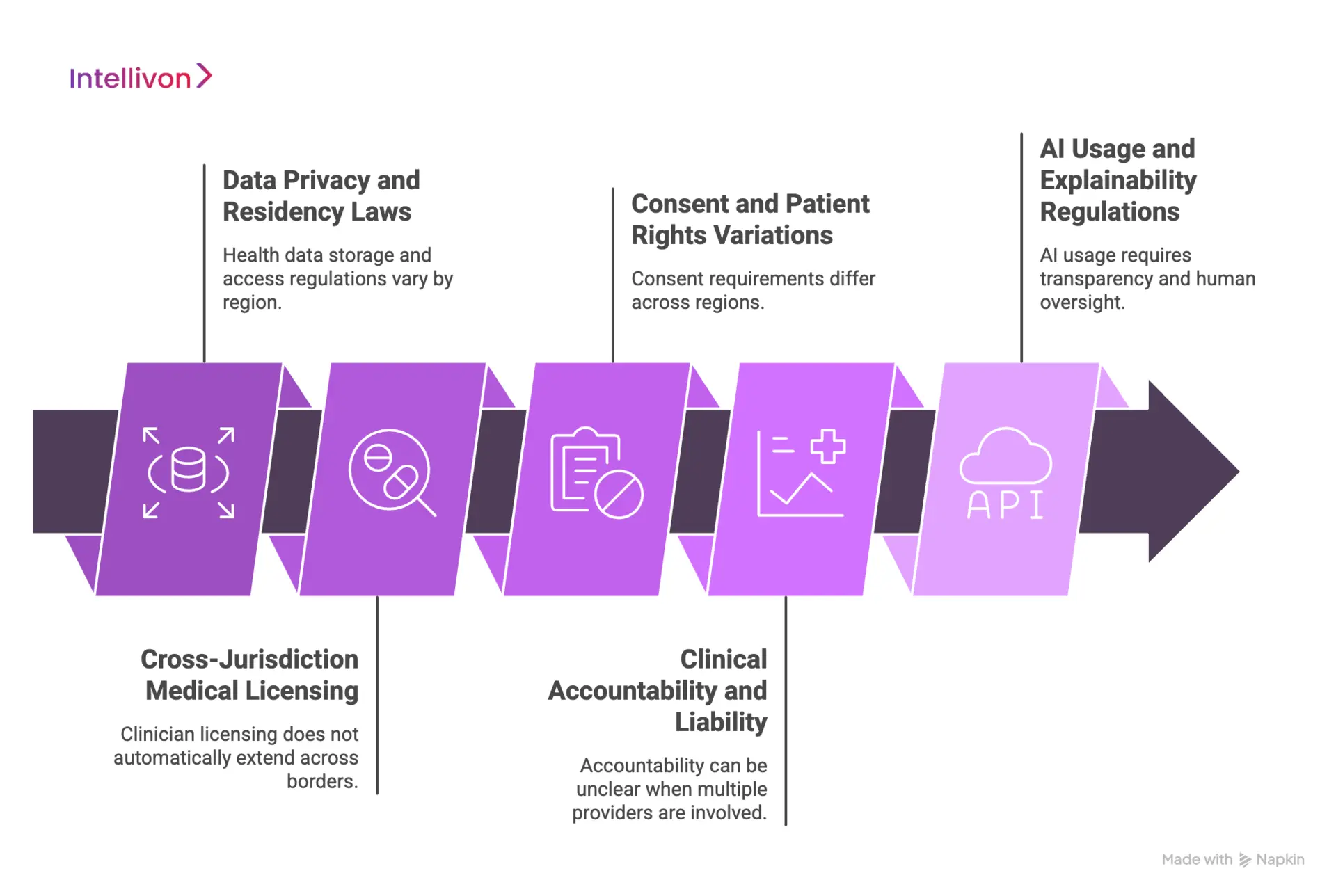
1. Data Privacy and Residency Laws
The challenge:
Health data cannot always move freely across borders. Regulations such as GDPR, HIPAA, and regional data residency laws impose strict controls on where patient data is stored, processed, and accessed. Many telehealth systems centralize data by default, which creates silent exposure once international care begins. Over time, this increases audit risk and limits expansion into new regions.
How it is fixed:
Cross-border platforms enforce data residency rules by design. Storage, access, and replication adjust based on patient location and jurisdiction. Longitudinal records remain usable without violating localization requirements. As a result, enterprises retain continuity while staying defensible under regional privacy laws.
2. Cross-Jurisdiction Medical Licensing
The challenge:
Clinician licensing does not automatically extend across borders. A provider eligible in one country may be prohibited from delivering care in another. Manual checks do not scale and often fail under volume. This exposes enterprises to regulatory action and invalidates clinical decisions retroactively.
How it is fixed:
Eligibility logic is enforced at the routing layer. The platform evaluates clinician credentials against patient location before care begins. Only compliant providers are surfaced. This removes manual verification while preserving regulatory alignment as care volumes grow.
3. Consent and Patient Rights Variations
The challenge:
Consent requirements vary widely across regions. What is acceptable in one country may be insufficient in another. Static consent flows break when care crosses borders, especially for data sharing, recordings, and secondary use. Missing or invalid consent creates downstream legal exposure.
How it is fixed:
Consent becomes dynamic and jurisdiction-aware. The platform adjusts consent language, capture method, and retention rules based on location and care type. Versioning ensures proof of consent remains audit-ready even as regulations evolve. This keeps patient rights protected without slowing care delivery.
4. Clinical Accountability and Liability
The challenge:
Cross-border care complicates responsibility. When multiple providers contribute across regions, accountability can become unclear. This creates legal ambiguity around duty of care, prescribing authority, and escalation decisions. Without structure, risk accumulates quietly.
How it is fixed:
Platforms preserve clinician attribution and decision ownership across encounters. Every action is logged, traceable, and linked to an eligible provider. Escalation paths remain explicit. As a result, accountability stays clear even when care spans jurisdictions.
5. AI Usage and Explainability Regulations
The challenge:
AI-assisted triage, recommendations, and automation introduce new regulatory scrutiny. Many regions now require transparency, explainability, and human oversight. Black-box decisioning increases compliance risk, especially in regulated healthcare workflows.
How it is fixed:
AI is deployed as decision support, not decision replacement. Models operate within defined boundaries and surface rationale alongside outputs. Human oversight remains mandatory for clinical actions. This approach aligns with emerging AI governance frameworks while preserving operational efficiency.
Taken together, these challenges define whether cross-border telehealth remains controllable at scale. When compliance is enforced through architecture rather than policy alone, enterprises can expand globally without increasing regulatory or clinical risk.
How We Design Compliance-First Cross-Border Telehealth Platforms
Cross-border telehealth cannot be “made compliant” after launch. The safest path is to design the platform so compliance controls run automatically inside workflows. Intellivon approaches this as a governed build, not a feature build, because global care fails when policy stays in documents instead of system behavior.
Below is the seven-step process used to deliver cross-border platforms that scale without expanding regulatory exposure.
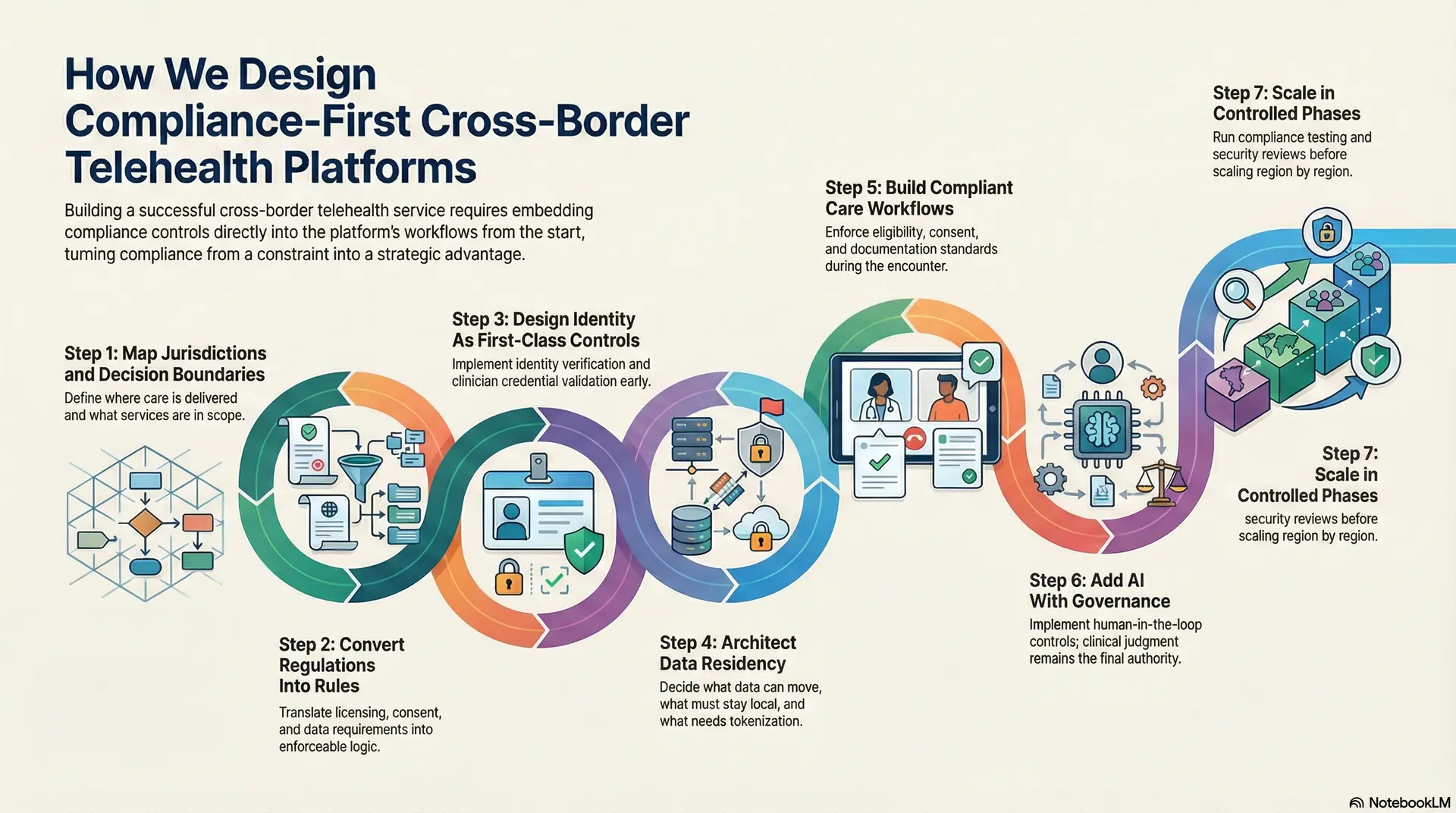
Step 1: Map Jurisdictions and Decision Boundaries
We start with clarity on where care will be delivered and what services are in scope. Define what qualifies as consultation, follow-up, triage, or second opinion in each region. In addition, lock decision boundaries for prescribing, escalation, and clinical responsibility.
This step prevents overreach and reduces downstream redesign. It also gives legal, compliance, and clinical leadership a shared operating map.
Step 2: Convert Regulations Into Rules
Translate licensing, prescribing, consent, and data residency requirements into enforceable logic. Build a policy model that can vary by country, state, and care type.
Therefore, the platform can change behavior automatically as geography changes. This eliminates manual checks that fail under scale. It also makes compliance measurable, not interpretive.
Step 3: Design Identity As First-Class Controls
Cross-border safety depends on knowing exactly who did what, and when. Implement identity verification, clinician credential validation, and role-based access control early.
Preserve clinician attribution across encounters so accountability never becomes vague. In addition, maintain longitudinal records so continuity survives travel and relocation. This is where many platforms break quietly, so Intellivon treats it as foundational.
Step 4: Architect Data Residency
Data localization cannot be solved with storage choices alone. Decide what data can move, what must stay local, and what requires tokenization or field-level controls. Then align integrations to those rules, including EHR connectivity and HL7 or FHIR exchange patterns.
As a result, clinicians get continuity without violating residency requirements. This also reduces duplication and data drift across regions.
Step 5: Build Compliant Care Workflows
Video and chat are table stakes. The real work sits in intake, triage, documentation, referrals, and follow-up ownership. Design workflows that enforce eligibility, consent validity, and documentation standards during the encounter.
In addition, make escalation paths explicit so risk is managed consistently. This keeps cross-border care predictable for operators and defensible for auditors.
Step 6: Add AI With Governance
AI can improve routing, triage support, and operational efficiency. However, it must operate within defined boundaries. Implement human-in-the-loop controls for clinical actions, and log AI inputs and outputs for audit.
Provide explainable reasoning where AI influences decisions. Intellivon applies AI as decision support, and clinical judgment remains the final authority. This approach improves speed without creating black-box risk.
Step 7: Scale in Controlled Phases
Before scaling, prove the platform under real workflows and regional constraints. Run compliance testing, security reviews, and audit log validation across jurisdictions. Then scale region by region with clear governance, ownership, and monitoring.
In addition, track leading risk indicators such as access anomalies, prescribing exceptions, and consent failures. This phased rollout keeps growth controlled while platform confidence increases.
Compliance-first design removes uncertainty from cross-border telehealth delivery. When controls are embedded into architecture and workflows, teams spend less time interpreting rules and more time delivering care.
The platform carries regulatory responsibility by default, which reduces friction as programs expand. This approach turns compliance from a constraint into a stabilizing advantage for global healthcare operations.
Cost to Build a Cross-Border Telehealth Platform
At Intellivon, cross-border telehealth platforms are built as a regulated global care infrastructure, not as extensions of domestic telemedicine tools. Cost planning is tied directly to jurisdictional risk, compliance exposure, and operational scale across regions. The objective is to invest deliberately in governance, continuity, and resilience so the platform performs reliably under real regulatory scrutiny. Budgets are structured to support sustained international operations, not pilot programs or region-specific workarounds.
When budget constraints exist, scope is refined collaboratively and strategically. However, regulatory, security, and governance requirements are never diluted. This approach balances cost discipline with long-term scalability and defensible ROI.
Estimated Phase-Wise Cost Breakdown
| Phase | Description | Estimated Cost Range (USD) |
| Discovery & Regulatory Mapping | Care model definition, jurisdiction analysis, licensing scope, compliance risk assessment, and KPI alignment | $8,000 – $15,000 |
| Architecture & Secure Platform Design | Multi-region architecture, data residency strategy, identity controls, resilience planning | $10,000 – $18,000 |
| Workflow & Governance Design | Cross-border clinical workflows, consent logic, escalation paths, and accountability mapping | $9,000 – $16,000 |
| Backend & Integration Development | EHR, pharmacy, labs, identity providers, payments, insurer, or employer systems | $15,000 – $28,000 |
| Frontend & Role-Based Interfaces | Patient, clinician, and admin interfaces with localization and accessibility controls | $12,000 – $22,000 |
| Security & Privacy Engineering | Encryption, access control, audit logs, monitoring, zero-trust enforcement | $10,000 – $18,000 |
| Testing & Compliance Validation | Security testing, workflow validation, regional compliance checks, and audit readiness | $7,000 – $12,000 |
| Deployment & Multi-Region Scaling | Cloud deployment, observability, failover, performance tuning | $8,000 – $14,000 |
Total initial investment: $80,000 – $180,000
Ongoing maintenance and optimization: 15–20% of the initial build per year
Hidden Costs Enterprises Should Plan For
Even well-planned cross-border programs face pressure when hidden cost drivers are ignored. Planning for these early protects timelines, budgets, and regulatory posture as scale increases.
- Integration complexity rises with fragmented EHRs and regional third-party systems
- Compliance overhead grows due to recurring audits and evolving cross-border regulations
- Data governance requires continuous validation, mapping, and consent enforcement
- Cloud costs increase with real-time video, routing logic, and analytics workloads
- Change management includes clinician onboarding and compliance training across regions
- Monitoring and optimization remain ongoing as policies, volume, and risk evolve
Best Practices to Avoid Budget Overruns
Based on Intellivon’s enterprise healthcare delivery experience, these practices consistently lead to predictable costs and controlled growth.
- Start with a focused geography and care scope before expanding
- Embed jurisdiction rules directly into architecture and workflows
- Use modular components that can be reused across regions and care programs
- Balance real-time services with asynchronous workflows to control cloud spend
- Maintain continuous observability across performance, security, and compliance
- Plan for regulatory evolution rather than one-time certification
Request a tailored proposal from Intellivon’s healthcare team to receive a delivery roadmap aligned with your budget, compliance priorities, and long-term cross-border growth strategy.

Security Considerations for Global Telehealth Platforms
Security becomes more complex once telehealth operates across regions. Data travels farther, access points multiply, and regulatory expectations rise. As a result, security must be designed as an always-on control layer rather than a checklist activity.
The considerations below reflect how enterprises protect patients, clinicians, and the organization as the global scale increases.
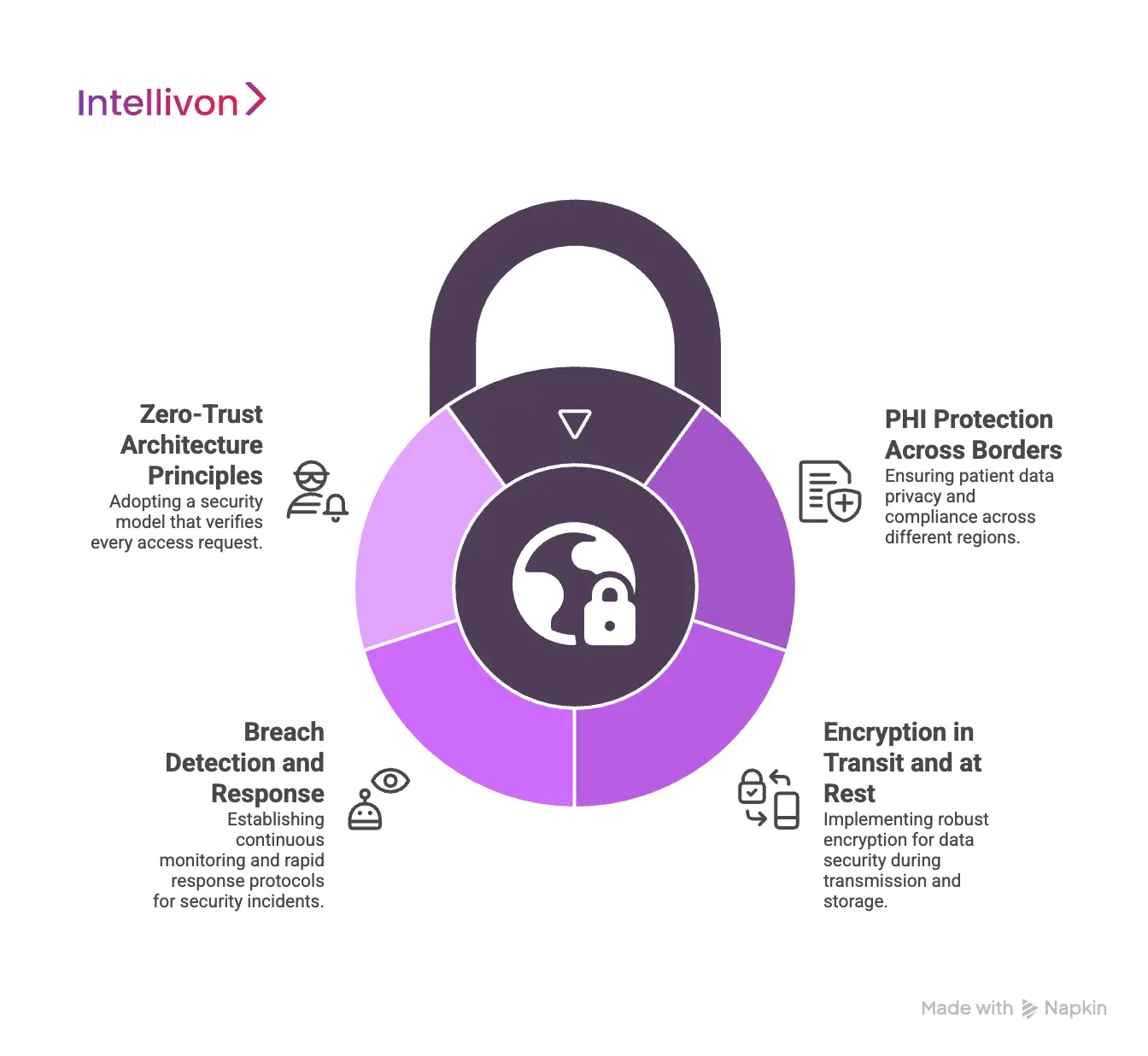
1. PHI Protection Across Borders
Protected health information carries different legal obligations depending on where it is accessed and stored. Cross-border platforms must assume that data will be viewed from multiple regions, even when storage remains localized. Therefore, access controls must evaluate user role, location, and purpose every time data is requested.
Field-level protections and segmentation reduce exposure if credentials are misused. When PHI protection is consistent across borders, enterprises avoid silent risk accumulation.
2. Encryption in Transit and at Rest
Encryption is the baseline, not a differentiator. Data must remain encrypted while moving between regions and while stored in any environment. However, cross-border platforms also need strong key management practices that respect regional requirements.
Centralized encryption without proper key separation creates concentration risk. By managing keys securely and regionally, platforms reduce blast radius and improve audit defensibility.
3. Breach Detection and Response
Global platforms increase the attack surface, which makes early detection critical. Security monitoring must operate continuously across regions, systems, and user roles.
Anomalous access patterns, unusual prescribing activity, or unexpected data exports should trigger alerts quickly. In addition, response workflows must be predefined and tested. When incidents occur, speed and clarity matter more than perfection.
4. Zero-Trust Architecture Principles
Cross-border telehealth cannot rely on perimeter-based security. Zero-trust principles assume no request is trusted by default, even from internal systems. Every access is verified, logged, and evaluated against policy.
This approach limits lateral movement and reduces insider risk. As platforms scale, zero-trust design allows security to grow without becoming brittle or overly restrictive.
When security is treated as a foundational capability, global telehealth platforms gain resilience. Enterprises protect patient trust while enabling international care delivery to scale with confidence.
Conclusion
Cross-border telehealth is becoming a core capability for healthcare enterprises operating across regions, workforces, and patient populations. However, success requires platforms designed for regulatory reality, clinical accountability, and operational scale.
When compliance, security, and continuity are embedded into architecture, global care becomes predictable rather than risky. This is where strategy meets execution. Intellivon helps enterprises design and build compliance-first cross-border telehealth platforms that scale safely and perform in real-world conditions. If global care is part of your growth roadmap, now is the moment to build it right.
Build a Cross-Border Telehealth Platform With Intellivon
At Intellivon, cross-border telehealth platforms are built as regulated global care systems, not extensions of domestic telemedicine tools. Every architectural decision is shaped by jurisdictional risk, clinical responsibility, and long-term enterprise use rather than speed of rollout.
Each platform is engineered for real healthcare environments where regulatory tolerance is low and oversight is constant.
As platforms expand across regions, specialties, or care models, governance and performance remain predictable. This approach enables global care delivery without increasing clinical, regulatory, or operational exposure.
Why Partner With Intellivon?
- Enterprise-grade cross-border architecture aligned with regulated international care delivery
- Proven integration expertise across EHRs, labs, pharmacies, insurers, and identity systems
- Compliance-by-design delivery supporting HIPAA, GDPR, data residency, and audit readiness
- Secure role-based access control with full action-level traceability across regions
- AI-assisted routing, triage, and workflow support with mandatory human oversight
- Cloud-native, multi-region infrastructure built for resilience and controlled expansion
- Modular delivery model enabling phased rollout by geography or care type
- Continuous optimization across performance, compliance, and adoption metrics
Book a strategy call to explore how a cross-border telehealth platform can operate as a trusted global care delivery layer, with Intellivon as your long-term technology and compliance partner.
FAQs
Q1. Is cross-border telehealth legal?
A1. Yes, cross-border telehealth is legal in many regions, but only when delivered within defined regulatory boundaries. Legality depends on patient location, clinician licensing, care type, and data handling practices.
Regulators generally allow cross-border care when it extends existing clinical relationships or supports follow-up and second opinions. Platforms must enforce jurisdiction-specific rules automatically. Without this, otherwise legal care can become non-compliant at scale.
Q2. How do licensing rules work internationally?
A2. Medical licenses do not transfer automatically across borders. Clinicians must be eligible to provide care in the patient’s jurisdiction, even if they are licensed elsewhere.
Some regions allow limited cross-border practice for second opinions or follow-ups. Therefore, platforms must evaluate clinician eligibility in real time. Manual verification does not scale and often creates hidden risk.
Q3. Can patient data cross borders?
A3. Patient data can cross borders only under strict conditions. Regulations such as GDPR and regional data residency laws govern where data is stored, processed, and accessed. Some data may need to remain local, while other elements can be shared securely.
Compliance requires controlled access, encryption, and consent enforcement. Platforms must manage this dynamically to preserve continuity without violating localization rules.
Q4. How is compliance managed across regions?
A4. Compliance is managed through platform-level enforcement rather than policy alone. Jurisdiction-aware logic controls licensing, prescribing, consent, data access, and auditability.
As care crosses borders, workflows adapt automatically. This reduces reliance on training and manual checks. Enterprises gain defensibility because compliance becomes measurable and traceable.
Q5. What types of care are best suited for cross-border telehealth?
A5. Cross-border telehealth works best for second opinions, post-diagnosis follow-ups, chronic care support, mental health continuity, and expatriate or traveler care. These models benefit from existing clinical context and clear accountability.
Virtual-first emergency care is less suitable. Successful programs align care type with regulatory tolerance and platform governance.